A SNARE required for retrograde transport to the endoplasmic reticulum
- PMID: 12893879
- PMCID: PMC187870
- DOI: 10.1073/pnas.1734000100
A SNARE required for retrograde transport to the endoplasmic reticulum
Abstract
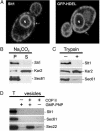
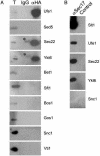
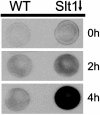
SNAREs (soluble N-ethylmaleimide-sensitive factor attachment protein receptors) are central components of the machinery mediating membrane fusion in all eukaryotic cells. Sequence analysis of the yeast genome revealed a previously uncharacterized SNARE, SNARE-like tail-anchored protein 1 (Slt1). Slt1 is an essential protein localized in the endoplasmic reticulum (ER). It forms a SNARE complex with Sec22 and the ER syntaxin Ufe1. Down-regulation of Slt1 levels leads to improper secretion of proteins normally resident in the ER. We suggest that Slt1 is a component of the SNAREpin required for retrograde traffic to the ER. Based on the previously reported association with Ufe1 and Sec22, Sec20 likely contributes the fourth SNARE to the SNAREpin.
Figures


References
-
- Söllner, T., Whiteheart, S. W., Brunner, M., Erdjument-Bromage, H., Geromanos, S., Tempst, P. & Rothman, J. E. (1993) Nature 362, 318–324. - PubMed
-
- Weber, T., Zemelman, B. V., McNew, J. A., Westermann, B., Gmachl, M., Parlati, F., Söllner, T. H. & Rothman, J. E. (1998) Cell 92, 759–772. - PubMed
-
- Sutton, R. B., Fasshauer, D., Jahn, R. & Brunger, A. T. (1998) Nature 395, 347–353. - PubMed
-
- Fukuda, R., McNew, J. A., Weber, T., Parlati, F., Engel, T., Nickel, W., Rothman, J. E. & Söllner, T. H. (2000) Nature 407, 198–202. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

