Mutation in the relA gene of Vibrio cholerae affects in vitro and in vivo expression of virulence factors
- PMID: 12896985
- PMCID: PMC166452
- DOI: 10.1128/JB.185.16.4672-4682.2003
Mutation in the relA gene of Vibrio cholerae affects in vitro and in vivo expression of virulence factors
Abstract
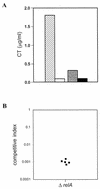
The relA gene product determines the level of (p)ppGpp, the effector nucleotides of the bacterial stringent response that are also involved in the regulation of other functions, like antibiotic production and quorum sensing. In order to explore the possible involvement of relA in the regulation of virulence of Vibrio cholerae, a relA homolog from the organism (relA(VCH)) was cloned and sequenced. The relA(VCH) gene encodes a 738-amino-acid protein having functions similar to those of other gram-negative bacteria, including Escherichia coli. A deltarelA::kan allele was generated by replacing approximately 31% of the open reading frame of wild-type relA of V. cholerae El Tor strain C6709 with a kanamycin resistance gene. The V. cholerae relA mutant strain thus generated, SHK17, failed to accumulate (p)ppGpp upon amino acid deprivation. Interestingly, compared to the wild type, C6709, the mutant strain SHK17 exhibited significantly reduced in vitro production of two principal virulence factors, cholera toxin (CT) and toxin-coregulated pilus (TCP), under virulence gene-inducing conditions. In vivo experiments carried out in rabbit ileal loop and suckling mouse models also confirmed our in vitro results. The data suggest that (p)ppGpp is essential for maximal expression of CT and TCP during in vitro growth, as well as during intestinal infection by virulent V. cholerae. Northern blot and reverse transcriptase PCR analyses indicated significant reduction in the transcript levels of both virulence factors in the relA mutant strain SHK17. Such marked alteration of virulence phenotypes in SHK17 appears most likely to be due to down regulation of transcript levels of toxR and toxT, the two most important virulence regulatory genes of V. cholerae. In SHK17, the altered expression of the two outer membrane porin proteins, OmpU and OmpT, indicated that the relA mutation most likely affects the ToxR-dependent virulence regulatory pathway, because it had been shown earlier that ToxR directly regulates their expression independently of ToxT.
Figures




References
-
- Ausubel, F. M., R. Brent, R. E. Kingston, D. D. Moore, J. G. Seidman, J. A. Smith, and K. Struhl (ed.). 1989. Current protocols in molecular biology. John Wiley and Sons, New York, N.Y.
-
- Bhadra, R. K., S. Roychoudhury, R. K. Banerjee, S. Kar, R. Majumdar, S. Sengupta, S. Chatterjee, G. Khetawat, and J. Das. 1995. Cholera toxin (CTX) genetic element in Vibrio cholerae O139. Microbiology 141:1977-1983. - PubMed
-
- Bik, E. M., A. E. Bunschoten, R. J. Willems, A. C. Chang, and F. R. Mooi. 1996. Genetic organization and functional analysis of the otn DNA essential for cell-wall polysaccharide synthesis in Vibrio cholerae O139. Mol. Microbiol. 20:799-811. - PubMed
-
- Cashel, M. 1969. The control of ribonucleic acid synthesis in Escherichia coli. J. Biol. Chem. 244:3133-3141. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

