Endothelial PDGF-B retention is required for proper investment of pericytes in the microvessel wall
- PMID: 12897053
- PMCID: PMC196228
- DOI: 10.1101/gad.266803
Endothelial PDGF-B retention is required for proper investment of pericytes in the microvessel wall
Abstract
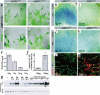
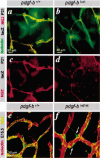
Several platelet-derived growth factor (PDGF) and vascular endothelial growth factor (VEGF) family members display C-terminal protein motifs that confer retention of the secreted factors within the pericellular space. To address the role of PDGF-B retention in vivo, we deleted the retention motif by gene targeting in mice. This resulted in defective investment of pericytes in the microvessel wall and delayed formation of the renal glomerulus mesangium. Long-term effects of lack of PDGF-B retention included severe retinal deterioration, glomerulosclerosis, and proteinuria. We conclude that retention of PDGF-B in microvessels is essential for proper recruitment and organization of pericytes and for renal and retinal function in adult mice.
Figures



References
-
- Allavena P., Dejana, E., Bussolino, F., Vecchi, A., and Mantovani, A. 1995. Cytokine regulation of endothelial cells. In Cytokines: A practical approach (ed. F.R. Balkwill), pp. 225-245. IRL Press, Oxford.
-
- Allt G. and Lawrenson, J.G. 2001. Pericytes: Cell biology and pathology. Cells Tissues Organs 169: 1-11. - PubMed
-
- Andersson M., Ostman, A., Westermark, B., and Heldin, C.H. 1994. Characterization of the retention motif in the C-terminal part of the long splice form of platelet-derived growth factor A-chain. J. Biol. Chem. 269: 926-9330. - PubMed
-
- Baeg G.H. and Perrimon, N. 2000. Functional binding of secreted molecules to heparin sulphate proteoglycans in Drosophila. Curr. Opin. Cell Biol. 12: 575-580. - PubMed
-
- Bussolino F., De Rossi, M., Sica, A., Colotta, F., Wang, J.M., Bocchietto, E., Padura, I.M., Bosia, A., Dejana, E., and Mantovani, A. 1991. Murine endothelioma cell lines transformed by polyoma middle T oncogene as target for and producers of cytokines. J. Immunol. 147: 2122-2129. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
