Identification of a unique core domain of par-4 sufficient for selective apoptosis induction in cancer cells
- PMID: 12897127
- PMCID: PMC166354
- DOI: 10.1128/MCB.23.16.5516-5525.2003
Identification of a unique core domain of par-4 sufficient for selective apoptosis induction in cancer cells
Abstract
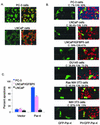
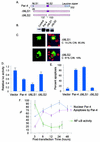
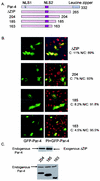
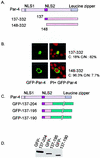
Recent studies indicated that the leucine zipper domain protein Par-4 induces apoptosis in certain cancer cells by activation of the Fas prodeath pathway and coparallel inhibition of NF-kappaB transcriptional activity. However, the intracellular localization or functional domains of Par-4 involved in apoptosis remained unknown. In the present study, structure-function analysis indicated that inhibition of NF-kappaB activity and apoptosis is dependent on Par-4 translocation to the nucleus via a bipartite nuclear localization sequence, NLS2. Cancer cells that were resistant to Par-4-induced apoptosis retained Par-4 in the cytoplasm. Interestingly, a 59-amino-acid core that included NLS2 but not the C-terminal leucine zipper domain was necessary and sufficient to induce Fas pathway activation, inhibition of NF-kappaB activity, and apoptosis. Most important, this core domain had an expanded target range for induction of apoptosis, extending to previously resistant cancer cells but not to normal cells. These findings have identified a unique death-inducing domain selective for apoptosis induction in cancer cells (SAC domain) which holds promise for identifying key differences between cancer and normal cells and for molecular therapy of cancer.
Figures




References
-
- Boghaert, E. R., S. F. Sells, A. J. Walid, P. Malone, N. M. Williams, M. H. Weinstein, R. Strange, and V. M. Rangnekar. 1997. Immunohistochemical analysis of the proapoptotic protein Par-4 in normal rat tissues. Cell Growth Differ. 8:881-890. - PubMed
-
- Boldin, M. P., E. E. Varfolomeev, Z. Pancer, I. L. Mett, J. H. Camonis, and D. Wallach. 1995. A novel protein that interacts with the death domain of Fas/APO1 contains a sequence motif related to the death domain. J. Biol. Chem. 270:7795-7798. - PubMed
-
- Chakraborty, M., S. G. Qiu, K. M. Vasudevan, and V. M. Rangnekar. 2001. Par-4 drives trafficking and activation of Fas and Fasl to induce prostate cancer cell apoptosis and tumor regression. Cancer Res. 61:7255-7263. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous
