Silencing of the novel p53 target gene Snk/Plk2 leads to mitotic catastrophe in paclitaxel (taxol)-exposed cells
- PMID: 12897130
- PMCID: PMC166320
- DOI: 10.1128/MCB.23.16.5556-5571.2003
Silencing of the novel p53 target gene Snk/Plk2 leads to mitotic catastrophe in paclitaxel (taxol)-exposed cells
Abstract
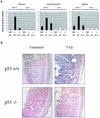
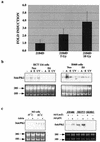
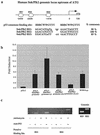
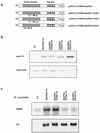
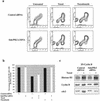
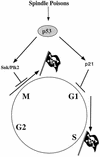
Loss of p53 sensitizes to antimicrotubule agents in human tumor cells, but little is known about its role during mitosis. We have identified the Polo-like kinase family member serum inducible kinase (Snk/Plk2) as a novel p53 target gene. Snk/Plk2 mutagenesis demonstrated that its kinase activity is negatively regulated by its C terminus. Small interfering RNA (siRNA)-mediated Snk/Plk2 silencing in the presence of the mitotic poisons paclitaxel (Taxol) or nocodazole significantly increased apoptosis, similar to p53 mutations, which confer paclitaxel sensitivity. Furthermore, we have demonstrated that the apoptosis due to silencing of Snk/Plk2 in the face of spindle damage occurs in mitotic cells and not in cells that have progressed to a G(1)-like state without dividing. Since siRNA directed against Snk/Plk2 promoted death of paclitaxel-treated cells in mitosis, we envision a mitotic checkpoint wherein p53-dependent activation of Snk/Plk2 prevents mitotic catastrophe following spindle damage. Finally, these studies suggest that disruption of Snk/Plk2 may be of therapeutic value in sensitizing paclitaxel-resistant tumors.
Figures



References
-
- Abrieu, A., T. Brassac, S. Galas, D. Fisher, J. C. Labbe, and M. Doree. 1998. The Polo-like kinase Plx1 is a component of the MPF amplification loop at the G2/M-phase transition of the cell cycle in Xenopus eggs. J. Cell Sci. 111:1751-1757. - PubMed
-
- Belge, G., L. Roque, J. Soares, S. Bruckmann, B. Thode, E. Fonseca, A. Clode, S. Bartnitzke, S. Castedo, and J. Bullerdiek. 1998. Cytogenetic investigations of 340 thyroid hyperplasias and adenomas revealing correlations between cytogenetic findings and histology. Cancer Genet. Cytogenet. 101:42-48. - PubMed
-
- Burns, T. F., E. J. Bernhard, and W. S. El-Deiry. 2001. Tissue specific expression of p53 target genes suggests a key role for KILLER/DR5 in p53-dependent apoptosis in vivo. Oncogene 20:4601-4612. - PubMed
-
- Burns, T. F., and W. S. El-Deiry. Microarray analysis of p53 target gene expression patterns in the spleen and thymus in response to ionizing radiation. Cancer Biol. Ther., in press. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous
