Potential role for ADAM15 in pathological neovascularization in mice
- PMID: 12897135
- PMCID: PMC166329
- DOI: 10.1128/MCB.23.16.5614-5624.2003
Potential role for ADAM15 in pathological neovascularization in mice
Abstract
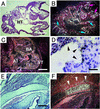
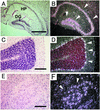
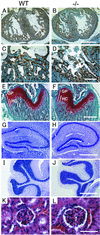
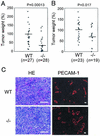
ADAM15 (named for a disintegrin and metalloprotease 15, metargidin) is a membrane-anchored glycoprotein that has been implicated in cell-cell or cell-matrix interactions and in the proteolysis of molecules on the cell surface or extracellular matrix. To characterize the potential roles of ADAM15 during development and in adult mice, we analyzed its expression pattern by mRNA in situ hybridization and generated mice carrying a targeted deletion of ADAM15 (adam15(-/-) mice). A high level of expression of ADAM15 was found in vascular cells, the endocardium, hypertrophic cells in developing bone, and specific areas of the hippocampus and cerebellum. However, despite the pronounced expression of ADAM15 in these tissues, no major developmental defects or pathological phenotypes were evident in adam15(-/-) mice. The elevated levels of ADAM15 in endothelial cells prompted an evaluation of its role in neovascularization. In a mouse model for retinopathy of prematurity, adam15(-/-) mice had a major reduction in neovascularization compared to wild-type controls. Furthermore, the size of tumors resulting from implanted B16F0 mouse melanoma cells was significantly smaller in adam15(-/-) mice than in wild-type controls. Since ADAM15 does not appear to be required for developmental angiogenesis or for adult homeostasis, it may represent a novel target for the design of inhibitors of pathological neovascularization.
Figures



References
-
- Abe, M., and Y. Sato. 2001. cDNA microarray analysis of the gene expression profile of VEGF-activated human umbilical vein endothelial cells. Angiogenesis 4:289-298. - PubMed
-
- Alfandari, D., H. Cousin, A. Gaultier, K. Smith, J. M. White, T. Darribere, and D. W. DeSimone. 2001. Xenopus ADAM 13 is a metalloprotease required for cranial neural crest-cell migration. Curr. Biol. 11:918-930. - PubMed
-
- Asakura, M., M. Kitakaze, S. Takashima, Y. Liao, F. Ishikura, T. Yoshinaka, H. Ohmoto, K. Node, K. Yoshino, H. Ishiguro, H. Asanuma, S. Sanada, Y. Matsumura, H. Takeda, S. Beppu, M. Tada, M. Hori, and S. Higashiyama. 2002. Cardiac hypertrophy is inhibited by antagonism of ADAM12 processing of HB-EGF: metalloproteinase inhibitors as a new therapy. Nat. Med. 8:35-40. - PubMed
-
- Black, R. A., C. T. Rauch, C. J. Kozlosky, J. J. Peschon, J. L. Slack, M. F. Wolfson, B. J. Castner, K. L. Stocking, P. Reddy, S. Srinivasan, N. Nelson, N. Boiani, K. A. Schooley, M. Gerhart, R. Davis, J. N. Fitzner, R. S. Johnson, R. J. Paxton, C. J. March, and D. P. Cerretti. 1997. A metalloproteinase disintegrin that releases tumour-necrosis factor-α from cells. Nature 385:729-733. - PubMed
-
- Black, R. A., and J. M. White. 1998. ADAMs: focus on the protease domain. Curr. Opin. Cell Biol. 10:654-659. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
