BMPER, a novel endothelial cell precursor-derived protein, antagonizes bone morphogenetic protein signaling and endothelial cell differentiation
- PMID: 12897139
- PMCID: PMC166349
- DOI: 10.1128/MCB.23.16.5664-5679.2003
BMPER, a novel endothelial cell precursor-derived protein, antagonizes bone morphogenetic protein signaling and endothelial cell differentiation
Abstract
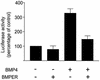
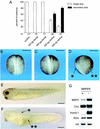
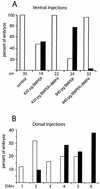
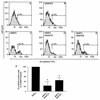
The development of endothelial cell precursors is essential for vasculogenesis. We screened for differentially expressed transcripts in endothelial cell precursors in developing mouse embryoid bodies. We cloned a complete cDNA encoding a protein that contains an amino-terminal signal peptide, five cysteine-rich domains, a von Willebrand D domain, and a trypsin inhibitor domain. We termed this protein BMPER (bone morphogenetic protein [BMP]-binding endothelial cell precursor-derived regulator). BMPER is specifically expressed in flk-1-positive cells and parallels the time course of flk-1 induction in these cells. In situ hybridization in mouse embryos demonstrates dorsal midline staining and staining of the aorto-gonadal-mesonephric region, which is known to host vascular precursor cells. BMPER is a secreted protein that directly interacts with BMP2, BMP4, and BMP6 and antagonizes BMP4-dependent Smad5 activation. In Xenopus embryos, ventral injection of BMPER mRNA results in axis duplication and downregulation of the expression of Xvent-1 (downstream target of Smad signaling). In an embryoid body differentiation assay, BMP4-dependent differentiation of endothelial cells in embryoid bodies is also antagonized by BMPER. Taken together, our data indicate that BMPER is a novel BMP-binding protein that is expressed by endothelial cell precursors, has BMP-antagonizing activity, and may play a role in endothelial cell differentiation by modulating local BMP activity.
Figures


Similar articles
-
Bmp2 antagonizes sonic hedgehog-mediated proliferation of cerebellar granule neurones through Smad5 signalling.Development. 2004 Jul;131(13):3159-68. doi: 10.1242/dev.01188. Development. 2004. PMID: 15197161
-
Menin is required for bone morphogenetic protein 2- and transforming growth factor beta-regulated osteoblastic differentiation through interaction with Smads and Runx2.J Biol Chem. 2004 Sep 24;279(39):40267-75. doi: 10.1074/jbc.M401312200. Epub 2004 May 18. J Biol Chem. 2004. PMID: 15150273
-
BMPER is an endothelial cell regulator and controls bone morphogenetic protein-4-dependent angiogenesis.Circ Res. 2008 Oct 10;103(8):804-12. doi: 10.1161/CIRCRESAHA.108.178434. Epub 2008 Sep 11. Circ Res. 2008. PMID: 18787191
-
Bone morphogenetic proteins.Growth Factors. 2004 Dec;22(4):233-41. doi: 10.1080/08977190412331279890. Growth Factors. 2004. PMID: 15621726 Review.
-
The role of bone morphogenetic protein signaling in vascular calcification.Bone. 2020 Dec;141:115542. doi: 10.1016/j.bone.2020.115542. Epub 2020 Jul 28. Bone. 2020. PMID: 32736145 Free PMC article. Review.
Cited by
-
TGF-β superfamily-induced transcriptional activation pathways establish the RAD52-dependent ALT machinery during malignant transformation of MPNSTs.Sci Rep. 2024 Nov 2;14(1):26475. doi: 10.1038/s41598-024-76732-z. Sci Rep. 2024. PMID: 39488637 Free PMC article.
-
Single-cell RNA-seq uncovered hemocyte functional subtypes and their differentiational characteristics and connectivity with morphological subpopulations in Litopenaeus vannamei.Front Immunol. 2022 Sep 13;13:980021. doi: 10.3389/fimmu.2022.980021. eCollection 2022. Front Immunol. 2022. PMID: 36177045 Free PMC article.
-
Successfully Managed Respiratory Insufficiency in a Patient with a Novel Pathogenic Variant of the BMPER Gene: A Case Report.Diagnostics (Basel). 2022 Mar 3;12(3):626. doi: 10.3390/diagnostics12030626. Diagnostics (Basel). 2022. PMID: 35328179 Free PMC article.
-
Shaping BMP morphogen gradients in the Drosophila embryo and pupal wing.Development. 2006 Jan;133(2):183-93. doi: 10.1242/dev.02214. Development. 2006. PMID: 16368928 Free PMC article. Review.
-
Development of the vertebral morphogenetic field in the mouse: interactions between Crossveinless-2 and Twisted Gastrulation.Dev Biol. 2008 Nov 1;323(1):6-18. doi: 10.1016/j.ydbio.2008.08.019. Epub 2008 Aug 29. Dev Biol. 2008. PMID: 18789316 Free PMC article.
References
-
- Adelman, C. A., S. Chattopadhyay, and J. J. Bieker. 2002. The BMP/BMPR/Smad pathway directs expression of the erythroid-specific EKLF and GATA1 transcription factors during embryoid body differentiation in serum-free media. Development 129:539-549. - PubMed
-
- Allen, S., A. M. Abuzenadah, J. L. Blagg, J. Hinks, I. M. Nesbitt, A. C. Goodeve, T. Gursel, J. Ingerslev, I. R. Peake, and M. E. Daly. 2000. Two novel type 2N von Willebrand disease-causing mutations that result in defective factor VIII binding, multimerization, and secretion of von Willebrand factor. Blood 95:2000-2007. - PubMed
-
- Balemans, W., and W. vanHul. 2002. Extracellular regulation of BMP signaling in vertebrates: a cocktail of modulators. Dev. Biol. 250:231-250. - PubMed
-
- Bautch, V. 2002. Embryonic stem cell differentiation and the vascular lineage. Humana Press, Totowa, N.J. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
