Polysialic acid directs tumor cell growth by controlling heterophilic neural cell adhesion molecule interactions
- PMID: 12897159
- PMCID: PMC166353
- DOI: 10.1128/MCB.23.16.5908-5918.2003
Polysialic acid directs tumor cell growth by controlling heterophilic neural cell adhesion molecule interactions
Abstract
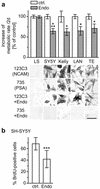
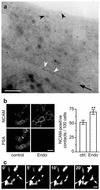
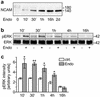
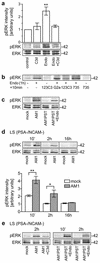
Polysialic acid (PSA), a carbohydrate polymer attached to the neural cell adhesion molecule (NCAM), promotes neural plasticity and tumor malignancy, but its mode of action is controversial. Here we establish that PSA controls tumor cell growth and differentiation by interfering with NCAM signaling at cell-cell contacts. Interactions between cells with different PSA and NCAM expression profiles were initiated by enzymatic removal of PSA and by ectopic expression of NCAM or PSA-NCAM. Removal of PSA from the cell surface led to reduced proliferation and activated extracellular signal-regulated kinase (ERK), inducing enhanced survival and neuronal differentiation of neuroblastoma cells. Blocking with an NCAM-specific peptide prevented these effects. Combinatorial transinteraction studies with cells and membranes with different PSA and NCAM phenotypes revealed that heterophilic NCAM binding mimics the cellular responses to PSA removal. In conclusion, our data demonstrate that PSA masks heterophilic NCAM signals, having a direct impact on tumor cell growth. This provides a mechanism for how PSA may promote the genesis and progression of highly aggressive PSA-NCAM-positive tumors.
Figures


Similar articles
-
The neural cell adhesion molecule NCAM regulates neuritogenesis by multiple mechanisms of interaction.Neurochem Int. 2006 Jul;49(1):1-11. doi: 10.1016/j.neuint.2005.12.011. Epub 2006 Feb 13. Neurochem Int. 2006. PMID: 16469417
-
A role for polysialic acid in neural cell adhesion molecule heterophilic binding to proteoglycans.J Biol Chem. 1998 Oct 16;273(42):27124-9. doi: 10.1074/jbc.273.42.27124. J Biol Chem. 1998. PMID: 9765230
-
[The impact of polysialic acid (PSA) and polysialylated neural cell adhesion molecule (PSA-NCAM) on tumor and cell signaling pathways].Yi Chuan. 2014 Aug;36(8):739-46. doi: 10.3724/SP.J.1005.2014.0739. Yi Chuan. 2014. PMID: 25143271 Review. Chinese.
-
Regulation of neural cell adhesion molecule polysialylation state by cell-cell contact and protein kinase C delta.J Neurosci Res. 2000 Sep 15;61(6):636-45. doi: 10.1002/1097-4547(20000915)61:6<636::AID-JNR7>3.0.CO;2-F. J Neurosci Res. 2000. PMID: 10972960
-
Polysialic acid-neural cell adhesion molecule in brain plasticity: from synapses to integration of new neurons.Brain Res Rev. 2007 Nov;56(1):101-18. doi: 10.1016/j.brainresrev.2007.05.014. Epub 2007 Jul 4. Brain Res Rev. 2007. PMID: 17658613 Review.
Cited by
-
The Graphical Studies of the Major Molecular Interactions for Neural Cell Adhesion Molecule (NCAM) Polysialylation by Incorporating Wenxiang Diagram into NMR Spectroscopy.Int J Mol Sci. 2022 Dec 1;23(23):15128. doi: 10.3390/ijms232315128. Int J Mol Sci. 2022. PMID: 36499451 Free PMC article.
-
The polysialylated neural cell adhesion molecule promotes neurogenesis in vitro.Neurochem Res. 2006 Feb;31(2):215-25. doi: 10.1007/s11064-005-9021-7. Epub 2006 Mar 30. Neurochem Res. 2006. PMID: 16572258
-
Structure and mutagenesis of neural cell adhesion molecule domains: evidence for flexibility in the placement of polysialic acid attachment sites.J Biol Chem. 2010 Aug 27;285(35):27360-27371. doi: 10.1074/jbc.M110.140038. Epub 2010 Jun 23. J Biol Chem. 2010. PMID: 20573953 Free PMC article.
-
Cell Adhesion Molecules and Ubiquitination-Functions and Significance.Biology (Basel). 2015 Dec 23;5(1):1. doi: 10.3390/biology5010001. Biology (Basel). 2015. PMID: 26703751 Free PMC article. Review.
-
Proinflammatory Macrophage Activation by the Polysialic Acid-Siglec-16 Axis Is Linked to Increased Survival of Patients with Glioblastoma.Clin Cancer Res. 2023 Jun 13;29(12):2266-2279. doi: 10.1158/1078-0432.CCR-22-1488. Clin Cancer Res. 2023. PMID: 37058255 Free PMC article.
References
-
- Aubert, I., J. L. Ridet, and F. H. Gage. 1995. Regeneration in the adult mammalian CNS: guided by development. Curr. Opin. Neurobiol. 5:625-635. - PubMed
-
- Cavallaro, U., J. Niedermeyer, M. Fuxa, and G. Christofori. 2001. N-CAM modulates tumour-cell adhesion to matrix by inducing fibroblast growth factor-receptor signalling. Nat. Cell Biol. 3:650-657. - PubMed
-
- Cole, G. J., and R. Akeson. 1989. Identification of a heparin binding domain of the neural cell adhesion molecule N-CAM with synthetic peptides. Neuron 2:1157-1165. - PubMed
-
- Crossin, K. L., and L. A. Krushel. 2000. Cellular signaling by neural cell adhesion molecules of the immunoglobulin superfamily. Dev. Dyn. 218:260-279. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous
