Support for a meiotic recombination initiation complex: interactions among Rec102p, Rec104p, and Spo11p
- PMID: 12897161
- PMCID: PMC166337
- DOI: 10.1128/MCB.23.16.5928-5938.2003
Support for a meiotic recombination initiation complex: interactions among Rec102p, Rec104p, and Spo11p
Abstract
Initiation of meiotic recombination in the yeast Saccharomyces cerevisiae requires at least 10 gene products. The initiation event creates double-strand breaks, which are then processed by other recombination enzymes. A variety of classical observations, such as the existence of recombination nodules, have suggested that the proteins catalyzing recombination form a complex. A variety of lines of evidence indicate that Rad50p, Mre11p, and Xrs2p interact, and genetic data suggesting interactions between Rec102p and Rec104p have been reported. It has recently been shown that Spo11p coimmunoprecipitates with Rec102p in meiosis as well. In this paper, we provide genetic and biochemical evidence that the meiosis-specific proteins Rec102p, Rec104p, and Spo11p all interact with each other in meiosis. Furthermore, we demonstrate that the interaction between Rec102p and Spo11p does not require Rec104p. Likewise, the interaction between Rec104p and Rec102p does not require Spo11p, although Spo11p may stabilize that association. The interactions suggest that Spo11p, Rec102p, and Rec104p may form a trimeric complex during the initiation of recombination.
Figures
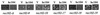


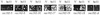
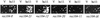


Similar articles
-
Functional interactions between SPO11 and REC102 during initiation of meiotic recombination in Saccharomyces cerevisiae.Genetics. 2002 Jan;160(1):111-22. doi: 10.1093/genetics/160.1.111. Genetics. 2002. PMID: 11805049 Free PMC article.
-
Identification of residues in yeast Spo11p critical for meiotic DNA double-strand break formation.Mol Cell Biol. 2002 Feb;22(4):1106-15. doi: 10.1128/MCB.22.4.1106-1115.2002. Mol Cell Biol. 2002. PMID: 11809802 Free PMC article.
-
HO endonuclease-induced recombination in yeast meiosis resembles Spo11-induced events.Proc Natl Acad Sci U S A. 2000 Dec 19;97(26):14500-5. doi: 10.1073/pnas.97.26.14500. Proc Natl Acad Sci U S A. 2000. PMID: 11121053 Free PMC article.
-
Modulating and targeting meiotic double-strand breaks in Saccharomyces cerevisiae.Methods Mol Biol. 2009;557:27-33. doi: 10.1007/978-1-59745-527-5_3. Methods Mol Biol. 2009. PMID: 19799174 Review.
-
Mechanism and control of meiotic recombination initiation.Curr Top Dev Biol. 2001;52:1-53. doi: 10.1016/s0070-2153(01)52008-6. Curr Top Dev Biol. 2001. PMID: 11529427 Review.
Cited by
-
Tethering recombination initiation proteins in Saccharomyces cerevisiae promotes double strand break formation.Genetics. 2009 Jun;182(2):447-58. doi: 10.1534/genetics.109.102640. Epub 2009 Mar 30. Genetics. 2009. PMID: 19332879 Free PMC article.
-
Saccharomyces cerevisiae Mer2, Mei4 and Rec114 form a complex required for meiotic double-strand break formation.Genetics. 2006 Aug;173(4):1969-81. doi: 10.1534/genetics.106.058768. Epub 2006 Jun 18. Genetics. 2006. PMID: 16783010 Free PMC article.
-
Both conserved and non-conserved regions of Spo11 are essential for meiotic recombination initiation in yeast.Mol Genet Genomics. 2006 Oct;276(4):313-21. doi: 10.1007/s00438-006-0143-7. Epub 2006 Jul 1. Mol Genet Genomics. 2006. PMID: 16816949
-
Structural and functional characterization of the Spo11 core complex.Nat Struct Mol Biol. 2021 Jan;28(1):92-102. doi: 10.1038/s41594-020-00534-w. Epub 2021 Jan 4. Nat Struct Mol Biol. 2021. PMID: 33398171 Free PMC article.
-
Factors affecting splicing strength of yeast genes.Comp Funct Genomics. 2011;2011:212146. doi: 10.1155/2011/212146. Epub 2011 Nov 20. Comp Funct Genomics. 2011. PMID: 22162666 Free PMC article.
References
-
- Alani, E., R. Padmore, and N. Kleckner. 1990. Analysis of wild-type and rad50 mutants of yeast suggests an intimate relationship between meiotic chromosome synapsis and recombination. Cell 61:419-436. - PubMed
-
- Ashley, T., and A. Plug. 1998. Caught in the act: deducing meiotic function from protein immunolocalization. Curr. Top. Dev. Biol. 37:201-239. - PubMed
-
- Batty, D. P., and R. D. Wood. 2000. Damage recognition in nucleotide excision repair of DNA. Gene 241:193-204. - PubMed
-
- Berben, G., J. Dumont, V. Gilliquet, P. A. Bolleand, and F. Hilger. 1991. The YDp plasmids: a uniform set of vectors bearing versatile gene disruption cassettes for Saccharomyces cerevisiae. Yeast 7:475-477. - PubMed
-
- Bergerat, A., B. de Massy, D. Gadelle, P. C. Varoutas, A. Nicolas, and P. Forterre. 1997. An atypical topoisomerase II from archaea with implications for meiotic recombination. Nature 386:414-417. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
