Dual action of hydrogen peroxide on synaptic transmission at the frog neuromuscular junction
- PMID: 12897166
- PMCID: PMC2343314
- DOI: 10.1113/jphysiol.2003.050690
Dual action of hydrogen peroxide on synaptic transmission at the frog neuromuscular junction
Abstract
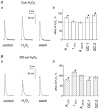
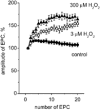
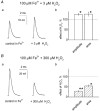
There is evidence that reactive oxygen species (ROS) are produced and released during neuromuscular activity, but their role in synaptic transmission is not known. Using a two-electrode voltage-clamp technique, at frog neuromuscular junctions, the action H2O2 on end-plate currents (EPC) was studied to determine the targets for this membrane-permeable ROS. In curarized or cut muscles, micromolar concentrations of H2O2 increased the amplitude of EPCs. Higher (> 30 microM) doses inhibited EPCs and prolonged current decay. These effects were presynaptic since H2O2 did not change the amplitude or duration of miniature EPCs (although it reduced the rate of spontaneous release at high concentrations). Quantal analysis and deconvolution methods showed that facilitation of EPCs was due to increased quantal release, while depression was accompanied by temporal dispersion of evoked release. Extracellular recordings revealed prolonged presynaptic Ca2+ entry in the presence of high H2O2. Both low and high H2O2 increased presynaptic potentiation during high-frequency stimulation. Pro-oxidant Fe2+ did not affect facilitation by low doses of H2O2 but augmented the inhibition of EPCs by high H2O2, indicating involvement of hydroxyl radicals. High Mg2+ and the ROS scavenger N-acetylcysteine eliminated both the facilitatory and depressant effects of H2O2. The facilitatory effect of H2O2 was prevented by protein kinase C (PKC) inhibitors and 4beta-phorbol 12-myristate, 13-acetate (PMA), an activator of PKC. PKC inhibitors but not PMA also abolished the depressant effect of H2O2. Our data suggest complex presynaptic actions of H2O2, which could serve as a fast feedback modulator of intense neuromuscular transmission.
Figures
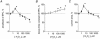
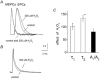
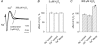
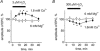
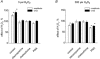
References
-
- Barstad JA, Lilleheil G. Transversaly cut diaphragm preparation from rat. An adjuvant tool in the study of the physiology and pharmacology of the myoneural junction. Arch Int Pharmacodyn Ther. 1968;175:373–390. - PubMed
-
- Castonguay A, Lévesque S, Robitaille R. Glial cells as active partners in synaptic functions. Prog Brain Res. 2001;132:227–240. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

