Cold exposure induces tissue-specific modulation of the insulin-signalling pathway in Rattus norvegicus
- PMID: 12897167
- PMCID: PMC2343313
- DOI: 10.1113/jphysiol.2003.050369
Cold exposure induces tissue-specific modulation of the insulin-signalling pathway in Rattus norvegicus
Abstract
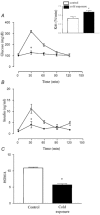
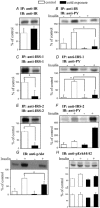
Cold exposure provides a reproducible model of improved glucose turnover accompanied by reduced steady state and glucose-induced insulin levels. In the present report we performed immunoprecipitation and immunoblot studies to evaluate the initial and intermediate steps of the insulin-signalling pathway in white and brown adipose tissues, liver and skeletal muscle of rats exposed to cold. Basal and glucose-induced insulin secretion were significantly impaired, while glucose clearance rates during a glucose tolerance test and the constant for glucose decay during a 15 min insulin tolerance test were increased, indicating a significantly improved glucose turnover and insulin sensitivity in rats exposed to cold. Evaluation of protein levels and insulin-induced tyrosine (insulin receptor, insulin receptor substrates (IRS)-1 and -2, ERK (extracellular signal-related kinase)) or serine (Akt; protein kinase B) phosphorylation of proteins of the insulin signalling cascade revealed a tissue-specific pattern of regulation of the molecular events triggered by insulin such that in white adipose tissue and skeletal muscle an impaired molecular response to insulin was detected, while in brown adipose tissue an enhanced response to insulin was evident. In muscle and white and brown adipose tissues, increased 2-deoxy-D-glucose (2-DG) uptake was detected. Thus, during cold exposure there is a tissue-specific regulation of the insulin-signalling pathway, which seems to favour heat-producing brown adipose tissue. Nevertheless, muscle and white adipose tissue are able to take up large amounts of glucose, even in the face of an apparent molecular resistance to insulin.
Figures








References
-
- Araujo EP, Amaral ME, Souza CT, Bordin S, Ferreira F, Saad MJ, Boschero AC, Magalhaes EC, Velloso LA. Blockade of IRS1 in isolated rat pancreatic islets improves glucose-induced insulin secretion. FEBS Lett. 2002;531:437–442. - PubMed
-
- Bonora E, Zavaroni I, Alpi O, Pezzarossa A, Dall'Aglio E, Coscelli C, Butturini U. Influence of the menstrual cycle on glucose tolerance and insulin secretion. Am J Obstet Gynecol. 1987;157:140–141. - PubMed
-
- Cassis L, Laughter A, Fettinger M, Akers S, Speth R, Burke G, King V, Dwoskin L. Cold exposure regulates the renin-angiotensin system. J Pharmacol Exp Ther. 1998;286:718–726. - PubMed
-
- Cunningham JJ, Gulino MA, Meara PA, Bode HH. Enhanced hepatic insulin sensitivity and peripheral glucose uptake in cold acclimating rats. Endocrinology. 1985;117:1585–1589. - PubMed
-
- Dulloo AG, Young JB, Landsberg L. Sympathetic nervous system responses to cold exposure and diet in rat skeletal muscle. Am J Physiol. 1988;255:E180–188. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

