Adenosine mediates IL-13-induced inflammation and remodeling in the lung and interacts in an IL-13-adenosine amplification pathway
- PMID: 12897202
- PMCID: PMC166289
- DOI: 10.1172/JCI16815
Adenosine mediates IL-13-induced inflammation and remodeling in the lung and interacts in an IL-13-adenosine amplification pathway
Abstract
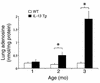
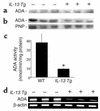
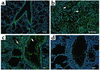
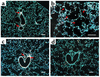
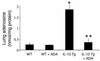
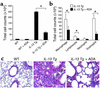
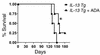
IL-13 is an important mediator of inflammation and remodeling. We hypothesized that adenosine accumulation, alterations in adenosine receptors, and adenosine-IL-13 autoinduction are critical events in IL-13-induced pathologies. To test this, we characterized the effects of IL-13 overexpression on the levels of adenosine, adenosine deaminase (ADA) activity, and adenosine receptors in the murine lung. We also determined whether adenosine induced IL-13 in lungs from ADA-null mice. IL-13 induced an inflammatory and remodeling response that caused respiratory failure and death. During this response, IL-13 caused a progressive increase in adenosine accumulation, inhibited ADA activity and mRNA accumulation, and augmented the expression of the A1, A2B, and A3 but not the A2A adenosine receptors. ADA enzyme therapy diminished the IL-13-induced increase in adenosine, inhibited IL-13-induced inflammation, chemokine elaboration, fibrosis, and alveolar destruction, and prolonged the survival of IL-13-transgenic animals. In addition, IL-13 was strongly induced by adenosine in ADA-null mice. These findings demonstrate that adenosine and adenosine signaling contribute to and influence the severity of IL-13-induced tissue responses. They also demonstrate that IL-13 and adenosine stimulate one another in an amplification pathway that may contribute to the nature, severity, progression, and/or chronicity of IL-13 and/or Th2-mediated disorders.
Figures


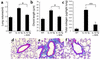

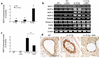
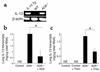
Comment in
-
IL-13 and adenosine: partners in a molecular dance?J Clin Invest. 2003 Aug;112(3):329-31. doi: 10.1172/JCI19392. J Clin Invest. 2003. PMID: 12897201 Free PMC article.
References
-
- Bradding, P., Redington, A.E., and Holgate, S.T. 1997. Airway wall remodelling in the pathogenesis of asthma: cytokine expression in the airways. In Airway wall remodelling in asthma. A.G. Stewart, editor. CRC Press Inc. Boca Raton, Florida, USA. 29–63.
-
- Belperio JA, et al. Interaction of IL-13 and C10 in the pathogenesis of bleomycin-induced pulmonary fibrosis. Am. J. Respir. Cell Mol. Biol. 2002;27:419–427. - PubMed
-
- Cosio MG, Guerassimov A. Chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care Med. 1999;160(Suppl.):S21–S25. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

