P2Y12 regulates platelet adhesion/activation, thrombus growth, and thrombus stability in injured arteries
- PMID: 12897207
- PMCID: PMC166294
- DOI: 10.1172/JCI17864
P2Y12 regulates platelet adhesion/activation, thrombus growth, and thrombus stability in injured arteries
Abstract
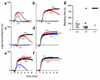
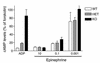
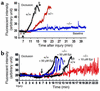
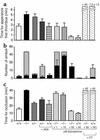
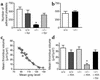
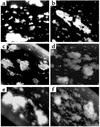
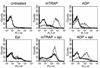
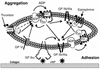
The critical role for ADP in arterial thrombogenesis was established by the clinical success of P2Y12 antagonists, currently used at doses that block 40-50% of the P2Y12 on platelets. This study was designed to determine the role of P2Y12 in platelet thrombosis and how its complete absence affects the thrombotic process. P2Y12-null mice were generated by a gene-targeting strategy. Using an in vivo mesenteric artery injury model and real-time continuous analysis of the thrombotic process, we observed that the time for appearance of first thrombus was delayed and that only small, unstable thrombi formed in P2Y12-/- mice without reaching occlusive size, in the absence of aspirin. Platelet adhesion to vWF was impaired in P2Y12-/- platelets. While adhesion to fibrinogen and collagen appeared normal, the platelets in thrombi from P2Y12-/- mice on collagen were less dense and less activated than their WT counterparts. P2Y12-/- platelet activation was also reduced in response to ADP or a PAR-4-activating peptide. Thus, P2Y12 is involved in several key steps of thrombosis: platelet adhesion/activation, thrombus growth, and stability. The data suggest that more aggressive strategies of P2Y12 antagonism will be antithrombotic without the requirement of aspirin cotherapy and may provide benefits even to the aspirin-nonresponder population.
Figures

Similar articles
-
Molecular identification and characterization of the platelet ADP receptor targeted by thienopyridine antithrombotic drugs.J Clin Invest. 2001 Jun;107(12):1591-8. doi: 10.1172/JCI12242. J Clin Invest. 2001. PMID: 11413167 Free PMC article.
-
P2Y12 receptor in platelet activation.Platelets. 2011;22(1):56-60. doi: 10.3109/09537104.2010.497231. Platelets. 2011. PMID: 21231822 Review.
-
Anticoagulants (thrombin inhibitors) and aspirin synergize with P2Y12 receptor antagonism in thrombosis.Circulation. 2003 Nov 25;108(21):2697-703. doi: 10.1161/01.CIR.0000093279.36628.12. Epub 2003 Nov 3. Circulation. 2003. PMID: 14597584
-
In vivo blockade of platelet ADP receptor P2Y12 reduces embolus and thrombus formation but not thrombus stability.Arterioscler Thromb Vasc Biol. 2003 Mar 1;23(3):518-23. doi: 10.1161/01.ATV.0000057809.32354.22. Epub 2003 Jan 23. Arterioscler Thromb Vasc Biol. 2003. PMID: 12615691
-
The role of ADP receptors in platelet function.Front Biosci. 2006 May 1;11:1977-86. doi: 10.2741/1939. Front Biosci. 2006. PMID: 16368572 Review.
Cited by
-
Central role of the P2Y12 receptor in platelet activation.J Clin Invest. 2004 Feb;113(3):340-5. doi: 10.1172/JCI20986. J Clin Invest. 2004. PMID: 14755328 Free PMC article. Review.
-
The active metabolite of Clopidogrel disrupts P2Y12 receptor oligomers and partitions them out of lipid rafts.Proc Natl Acad Sci U S A. 2006 Jul 18;103(29):11069-74. doi: 10.1073/pnas.0510446103. Epub 2006 Jul 11. Proc Natl Acad Sci U S A. 2006. PMID: 16835302 Free PMC article.
-
Role of Gas6 receptors in platelet signaling during thrombus stabilization and implications for antithrombotic therapy.J Clin Invest. 2005 Feb;115(2):237-46. doi: 10.1172/JCI22079. J Clin Invest. 2005. PMID: 15650770 Free PMC article.
-
Reduction in Subtypes and Sizes of Myocardial Infarction With Ticagrelor in PEGASUS-TIMI 54.J Am Heart Assoc. 2018 Nov 20;7(22):e009260. doi: 10.1161/JAHA.118.009260. J Am Heart Assoc. 2018. PMID: 30571502 Free PMC article. Clinical Trial.
-
Aspirin resistance.Adv Hematol. 2009;2009:937352. doi: 10.1155/2009/937352. Epub 2009 Apr 14. Adv Hematol. 2009. PMID: 19960045 Free PMC article.
References
-
- Hollopeter G, et al. Identification of the platelet ADP receptor targeted by antithrombotic drugs. Nature. 2001;409:202–207. - PubMed
-
- Gachet C. ADP receptors of platelets and their inhibition. Thromb. Haemost. 2001;86:222–232. - PubMed
-
- Gurbel PA, Malinin AI, Callahan KP, Serebruany VL, O’Connor CM. Effect of loading with clopidogrel at the time of coronary stenting on platelet aggregation and glycoprotein IIb/IIIa expression and platelet-leukocyte aggregate formation. Am. J. Cardiol. 2002;90:312–315. - PubMed
-
- CAPRIE Steering Committee. A randomised, blinded, trial of clopidogrel versus aspirin in patients at risk of ischaemic events (CAPRIE). CAPRIE Steering Committee. Lancet. 1996;348:1329–1339. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

