Sex-peptide is the molecular basis of the sperm effect in Drosophila melanogaster
- PMID: 12897240
- PMCID: PMC187889
- DOI: 10.1073/pnas.1631700100
Sex-peptide is the molecular basis of the sperm effect in Drosophila melanogaster
Abstract
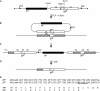
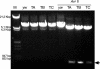
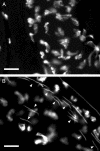
Mating elicits two major changes in the reproductive behavior of many insect females. The egg-laying rate increases and the readiness to accept males (receptivity) is reduced. These postmating responses last approximately 1 week in Drosophila melanogaster. Males that do not transfer sperm but transfer seminal fluid during mating induce a short-term response of 1 day. The long-term response of 1 week requires the presence of sperm (sperm effect). Hence, sperm is essential for the long-term persistence of the postmating responses. Three seminal fluid peptides elicit postmating responses: ovulin, sex-peptide (SP), and DUP99B. Using the technique of targeted mutagenesis by homologous recombination, we have produced males with mutant SP genes. Here, we report that males lacking functional SP elicit only a weak short-term response. However, these males do transfer sperm. Thus, (i) SP is the major agent eliciting the short-term and the long-term postmating responses and (ii) sperm is merely the carrier for SP. The second conclusion is supported by the finding that SP binds to sperm. The 36-aa-encoding SP gene is the first small Drosophila gene knocked out with the method of homologous recombination.
Figures


Comment in
-
Sex peptide and the sperm effect in Drosophila melanogaster.Proc Natl Acad Sci U S A. 2003 Aug 19;100(17):9643-4. doi: 10.1073/pnas.1834127100. Epub 2003 Aug 11. Proc Natl Acad Sci U S A. 2003. PMID: 12913117 Free PMC article. No abstract available.
References
-
- Chapman, T. (2001) Heredity 87, 511–521. - PubMed
-
- Gillott, C. (2003) Annu. Rev. Entomol. 48, 163–184. - PubMed
-
- Kubli, E. (1996) Adv. Dev. Biochem. 4, 99–128.
-
- Wolfner, M. (1997) Insect Biochem. Mol. Biol. 27, 179–192. - PubMed
-
- Chapman, T., Liddle, L. F., Kalb, J. M., Wolfner, M. F. & Partridge, L. (1995) Nature 373, 241–244. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

