The MSP1 gene is necessary to restrict the number of cells entering into male and female sporogenesis and to initiate anther wall formation in rice
- PMID: 12897248
- PMCID: PMC167165
- DOI: 10.1105/tpc.012401
The MSP1 gene is necessary to restrict the number of cells entering into male and female sporogenesis and to initiate anther wall formation in rice
Abstract
The function of the novel gene MSP1 (MULTIPLE SPOROCYTE), which controls early sporogenic development, was elucidated by characterizing a retrotransposon-tagged mutation of rice. The MSP1 gene encoded a Leu-rich repeat receptor-like protein kinase. The msp1 mutation gave rise to an excessive number of both male and female sporocytes. In addition, the formation of anther wall layers was disordered and the tapetum layer was lost completely. Although the mutation never affected homologous chromosome pairing and chiasma maintenance, the development of pollen mother cells was arrested at various stages of meiotic prophase I, which resulted in complete male sterility. Meanwhile, plural megaspore mother cells in a mutant ovule generated several megaspores, underwent gametogenesis, and produced germinable seeds when fertilized with wild-type pollen despite disorganized female gametophytes. In situ expression of MSP1 was detected in surrounding cells of male and female sporocytes and some flower tissues, but never in the sporocytes themselves. These results suggest that the MSP1 product plays crucial roles in restricting the number of cells entering into male and female sporogenesis and in initiating anther wall formation in rice.
Figures

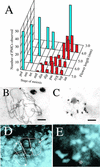


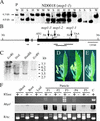

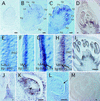
References
-
- Bhojwani, S.S., and Bhatnagar, S.P. (1992). The Embryology of Angiosperms. (New Delhi: Vikas Publishing House).
-
- Canales, C., Bhatt, A.M., Scott, R., and Dickinson, H. (2002). EXS, a putative LRR receptor kinase, regulates male germline cell number and tapetal identity and promotes seed development in Arabidopsis. Curr. Biol. 12, 1718–1727. - PubMed
-
- Chapman, G.P. (1987). The tapetum. Int. Rev. Cytol. 107, 111–125.
-
- Chaubal, R., Zanella, C., Trimnell, M.R., Fox, T.W., Albertsen, M.C., and Bedinger, P. (2000). Two male-sterile mutants of Zea mays (Poaceae) with an extra cell division in the anther wall. Am. J. Bot. 87, 1193–1201. - PubMed
-
- Clark, S.E. (2001). Cell signalling at the shoot meristem. Nat. Rev. Mol. Cell Biol. 2, 276–284. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

