Regulation and processing of maize histone deacetylase Hda1 by limited proteolysis
- PMID: 12897261
- PMCID: PMC167178
- DOI: 10.1105/tpc.013995
Regulation and processing of maize histone deacetylase Hda1 by limited proteolysis
Abstract
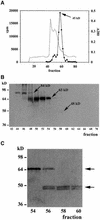
A maize histone deacetylase gene was identified as a homolog of yeast Hda1. The predicted protein corresponds to a previously purified maize deacetylase that is active as a protein monomer with a molecular weight of 48,000 and is expressed in all tissues of germinating embryos. Hda1 is synthesized as an enzymatically inactive protein with an apparent molecular weight of 84,000 that is processed to the active 48-kD form by proteolytic removal of the C-terminal part, presumably via a 65-kD intermediate. The enzymatically inactive 84-kD protein also is part of a 300-kD protein complex of unknown function. The proteolytic cleavage of ZmHda1 is regulated during maize embryo germination in vivo. Expression of the recombinant full-length protein and the 48-kD form confirmed that only the smaller enzyme form is active as a histone deacetylase. In line with this finding, we show that the 48-kD protein is able to repress transcription efficiently in a reporter gene assay, whereas the full-length protein, including the C-terminal part, lacks full repression activity. This report on the processing of Hda1-p84 to enzymatically active Hda1-p48 demonstrates that proteolytic cleavage is a mechanism to regulate the function of Rpd3/Hda1-type histone deacetylases.
Figures






Similar articles
-
Identification of maize histone deacetylase HD2 as an acidic nucleolar phosphoprotein.Science. 1997 Jul 4;277(5322):88-91. doi: 10.1126/science.277.5322.88. Science. 1997. PMID: 9204905
-
RPD3-type histone deacetylases in maize embryos.Biochemistry. 2000 Feb 22;39(7):1683-92. doi: 10.1021/bi9918184. Biochemistry. 2000. PMID: 10677216
-
Genetic characterisation of hda1+, a putative fission yeast histone deacetylase gene.Nucleic Acids Res. 1998 Jul 1;26(13):3247-54. doi: 10.1093/nar/26.13.3247. Nucleic Acids Res. 1998. PMID: 9628926 Free PMC article.
-
The origin and utility of histone deacetylases.FEBS Lett. 1997 Dec 15;419(2-3):157-60. doi: 10.1016/s0014-5793(97)01423-3. FEBS Lett. 1997. PMID: 9428625 Review.
-
The human histone deacetylase family.Exp Cell Res. 2001 Jan 15;262(2):75-83. doi: 10.1006/excr.2000.5080. Exp Cell Res. 2001. PMID: 11139331 Review.
Cited by
-
Relationship between DNA methylation and histone acetylation levels, cell redox and cell differentiation states in sugarbeet lines.Planta. 2006 Sep;224(4):812-27. doi: 10.1007/s00425-006-0267-3. Epub 2006 Apr 11. Planta. 2006. PMID: 16607556
-
Limited proteolysis of human histone deacetylase 1.BMC Biochem. 2006 Oct 5;7:22. doi: 10.1186/1471-2091-7-22. BMC Biochem. 2006. PMID: 17022812 Free PMC article.
-
Class II histone deacetylases: from sequence to function, regulation, and clinical implication.Mol Cell Biol. 2005 Apr;25(8):2873-84. doi: 10.1128/MCB.25.8.2873-2884.2005. Mol Cell Biol. 2005. PMID: 15798178 Free PMC article. Review. No abstract available.
-
Histone modifications and dynamic regulation of genome accessibility in plants.Curr Opin Plant Biol. 2007 Dec;10(6):645-52. doi: 10.1016/j.pbi.2007.07.013. Epub 2007 Sep 19. Curr Opin Plant Biol. 2007. PMID: 17884714 Free PMC article. Review.
-
Roles of dynamic and reversible histone acetylation in plant development and polyploidy.Biochim Biophys Acta. 2007 May-Jun;1769(5-6):295-307. doi: 10.1016/j.bbaexp.2007.04.007. Epub 2007 May 3. Biochim Biophys Acta. 2007. PMID: 17556080 Free PMC article. Review.
References
-
- Bertos, N.R., Wang, A.H., and Yang, Y.J. (2001). Class II histone deacetylases: Structure, functions, and regulations. Biochem. Cell Biol. 79, 243–252. - PubMed
-
- Bohner, S., Lenk, I.I., Rieping, M., Herold, M., and Gatz, C. (1999). Technical advances: Transcriptional activator TGV mediates dexamethasone-inducible and tetracycline-inactivable gene expression. Plant J. 19, 87–95. - PubMed
-
- Borchers, C., Peter, J.F., Hall, M.C., Kunkel, T.A., and Tomer, K.B. (2000). Identification of in-gel digested proteins by complementary peptide mass fingerprinting and tandem mass spectrometry data obtained on an electrospray ionization quadrupole time-of-flight mass spectrometer. Anal. Chem. 72, 1163–1168. - PubMed
-
- Boyer, L.A., Logie, C., Bonte, E., Becker, P.B., Wade, P.A., Wolffe, A.P., Wu, C., Imbalzano, A.N., and Peterson, C.L. (2000). Functional delineation of three groups of the ATP-dependent family of chromatin remodeling enzymes. J. Biol. Chem. 275, 18864–18870. - PubMed
-
- Bradford, M.M. (1976). A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 72, 248–254. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
LinkOut - more resources
Full Text Sources

