Integrin-alpha3 mediates binding of Chordin to the cell surface and promotes its endocytosis
- PMID: 12897802
- PMCID: PMC1326342
- DOI: 10.1038/sj.embor.embor902
Integrin-alpha3 mediates binding of Chordin to the cell surface and promotes its endocytosis
Abstract
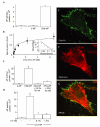
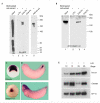
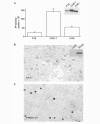
Dorsoventral patterning in animal development is regulated by a morphogenetic gradient of Bone morphogenetic protein signalling, which is established by a set of proteins that are conserved from Drosophila to vertebrates. These include Chordin (Chd)/Short gastrulation, Xolloid/Tolloid and Twisted gastrulation. Here, we report the identification of a cell-surface component of this morphogenetic pathway. Prompted by the observation that Chd protein bound to the surface of certain cell lines with subnanomolar affinity, we identified two cell-surface proteins that bind to Chd, one of which corresponds to Integrin-alpha3. Integrin-alpha3 and Chd are co-expressed in the Xenopus embryo. Transfection of Integrin-alpha3 increased the binding of Chd to the cell surface, which was competed by an excess of soluble Integrin-alpha3. After binding to the cell surface, Chd was translocated into intracellular endocytic compartments in a temperature-dependent manner. We propose that Integrin-alpha3 may regulate the concentration of Chd protein in the extracellular space by endocytosis.
Figures

References
-
- Araujo H., Negreiros E. & Bier E. ( 2003) Integrins modulate Sog activity in the Drosophila wing. Development, in the press. - PubMed
-
- Davis S. et al. . ( 1996) Isolation of angiopoietin-1, a ligand for the TIE2 receptor, by secretion-trap expression cloning. Cell, 87, 1161–1169. - PubMed
-
- Eldar A., Dorfman R., Weiss D., Ashe H., Shilo B.Z. & Barkai N. ( 2002) Robustness of the BMP morphogen gradient in Drosophila embryonic patterning. Nature, 419, 304–308. - PubMed
-
- Entchev E.V., Schwabedissen A. & González-Gaitán M. ( 2000) Gradient formation of the TGF-β homolog Dpp. Cell, 103, 981–991. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

