The VirD2 pilot protein of Agrobacterium-transferred DNA interacts with the TATA box-binding protein and a nuclear protein kinase in plants
- PMID: 12900506
- PMCID: PMC187781
- DOI: 10.1073/pnas.1733208100
The VirD2 pilot protein of Agrobacterium-transferred DNA interacts with the TATA box-binding protein and a nuclear protein kinase in plants
Abstract
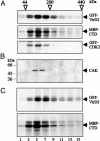
The bacterial virulence protein VirD2 plays an important role in nuclear import and chromosomal integration of Agrobacterium-transferred DNA in fungal, plant, animal, and human cells. Here we show that in nuclei of alfalfa cells, VirD2 interacts with and is phosphorylated by CAK2Ms, a conserved plant ortholog of cyclin-dependent kinase-activating kinases. CAK2Ms binds to and phosphorylates the C-terminal regulatory domain of RNA polymerase II largest subunit, which can recruit the TATA box-binding protein. VirD2 is found in tight association with the TATA box-binding protein in vivo. These results indicate that recognition of VirD2 is mediated by widely conserved nuclear factors in eukaryotes.
Figures



References
Publication types
MeSH terms
Substances
Associated data
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources

