Peroxisome proliferator-activated receptors alpha and gamma down-regulate allergic inflammation and eosinophil activation
- PMID: 12900517
- PMCID: PMC2194090
- DOI: 10.1084/jem.20021384
Peroxisome proliferator-activated receptors alpha and gamma down-regulate allergic inflammation and eosinophil activation
Abstract
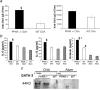
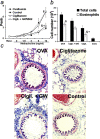
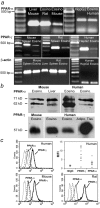
Allergic asthma is characterized by airway hyperresponsiveness, eosinophilia, and mucus accumulation and is associated with increased IgE concentrations. We demonstrate here that peroxisome proliferator-activated receptors (PPARs), PPAR-alpha and PPAR-gamma, which have been shown recently to be involved in the regulation of various cell types within the immune system, decrease antigen-induced airway hyperresponsiveness, lung inflammation, eosinophilia, cytokine production, and GATA-3 expression as well as serum levels of antigen-specific IgE in a murine model of human asthma. In addition, we demonstrate that PPAR-alpha and -gamma are expressed in eosinophils and their activation inhibits in vitro chemotaxis and antibody-dependent cellular cytotoxicity. Thus, PPAR-alpha and -gamma (co)agonists might be of therapeutic interest for the regulation of allergic or inflammatory reactions by targeting both regulatory and effector cells involved in the immune response.
Figures



References
-
- Wills-Karp, M. 1999. Immunologic basis of antigen-induced airway hyperresponsiveness. Annu. Rev. Immunol. 17:255–281. - PubMed
-
- Maddox, L., and D.A. Schwartz. 2002. The pathophysiology of asthma. Annu. Rev. Med. 53:477–498. - PubMed
-
- Dombrowicz, D., and M. Capron. 2001. Eosinophils, allergy and parasites. Curr. Opin. Immunol. 13:716–720. - PubMed
-
- Desvergne, B., and W. Wahli. 1999. Peroxisome proliferator-activated receptors: nuclear control of metabolism. Endocr. Rev. 20:649–688. - PubMed
-
- Devchand, P.R., H. Keller, J.M. Peters, M. Vazquez, F.J. Gonzalez, and W. Wahli. 1996. The PPAralpha-leukotriene B4 pathway to inflammation control. Nature. 384:39–43. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

