Purification and characterization of a novel mannitol dehydrogenase from a newly isolated strain of Candida magnoliae
- PMID: 12902227
- PMCID: PMC169128
- DOI: 10.1128/AEM.69.8.4438-4447.2003
Purification and characterization of a novel mannitol dehydrogenase from a newly isolated strain of Candida magnoliae
Abstract
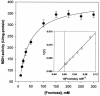
Mannitol biosynthesis in Candida magnoliae HH-01 (KCCM-10252), a yeast strain that is currently used for the industrial production of mannitol, is catalyzed by mannitol dehydrogenase (MDH) (EC 1.1.1.138). In this study, NAD(P)H-dependent MDH was purified to homogeneity from C. magnoliae HH-01 by ion-exchange chromatography, hydrophobic interaction chromatography, and affinity chromatography. The relative molecular masses of C. magnoliae MDH, as determined by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and size-exclusion chromatography, were 35 and 142 kDa, respectively, indicating that the enzyme is a tetramer. This enzyme catalyzed both fructose reduction and mannitol oxidation. The pH and temperature optima for fructose reduction and mannitol oxidation were 7.5 and 37 degrees C and 10.0 and 40 degrees C, respectively. C. magnoliae MDH showed high substrate specificity and high catalytic efficiency (k(cat) = 823 s(-1), K(m) = 28.0 mM, and k(cat)/K(m) = 29.4 mM(-1) s(-1)) for fructose, which may explain the high mannitol production observed in this strain. Initial velocity and product inhibition studies suggest that the reaction proceeds via a sequential ordered Bi Bi mechanism, and C. magnoliae MDH is specific for transferring the 4-pro-S hydrogen of NADPH, which is typical of a short-chain dehydrogenase reductase (SDR). The internal amino acid sequences of C. magnoliae MDH showed a significant homology with SDRs from various sources, indicating that the C. magnoliae MDH is an NAD(P)H-dependent tetrameric SDR. Although MDHs have been purified and characterized from several other sources, C. magnoliae MDH is distinguished from other MDHs by its high substrate specificity and catalytic efficiency for fructose only, which makes C. magnoliae MDH the ideal choice for industrial applications, including enzymatic synthesis of mannitol and salt-tolerant plants.
Figures


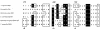

Similar articles
-
Purification and characterization of a novel erythrose reductase from Candida magnoliae.Appl Environ Microbiol. 2003 Jul;69(7):3710-8. doi: 10.1128/AEM.69.7.3710-3718.2003. Appl Environ Microbiol. 2003. PMID: 12839736 Free PMC article.
-
Molecular cloning and sequence analysis of a mannitol dehydrogenase gene and isolation of mdh promoter from Candida magnoliae.Biotechnol Lett. 2010 Aug;32(8):1089-94. doi: 10.1007/s10529-010-0257-1. Epub 2010 Apr 2. Biotechnol Lett. 2010. PMID: 20361235
-
Purification and characterization of a novel mannitol dehydrogenase from Lactobacillus intermedius.Biotechnol Prog. 2004 Mar-Apr;20(2):537-42. doi: 10.1021/bp034277p. Biotechnol Prog. 2004. PMID: 15059000
-
Kinetic study of the catalytic mechanism of mannitol dehydrogenase from Pseudomonas fluorescens.Biochemistry. 1999 Aug 10;38(32):10489-98. doi: 10.1021/bi990327g. Biochemistry. 1999. PMID: 10441145
-
Biotechnological production of mannitol and its applications.Appl Microbiol Biotechnol. 2011 Feb;89(4):879-91. doi: 10.1007/s00253-010-2979-3. Epub 2010 Nov 10. Appl Microbiol Biotechnol. 2011. PMID: 21063702 Review.
Cited by
-
Evidence for loss and reacquisition of alcoholic fermentation in a fructophilic yeast lineage.Elife. 2018 Apr 12;7:e33034. doi: 10.7554/eLife.33034. Elife. 2018. PMID: 29648535 Free PMC article.
-
Purification, characterization, gene cloning, and expression of a novel alcohol dehydrogenase with anti-prelog stereospecificity from Candida parapsilosis.Appl Environ Microbiol. 2007 Jun;73(11):3759-64. doi: 10.1128/AEM.02185-06. Epub 2007 Apr 13. Appl Environ Microbiol. 2007. PMID: 17435004 Free PMC article.
-
Cloning, purification and characterization of an NAD-Dependent D-Arabitol dehydrogenase from acetic acid bacterium, Acetobacter suboxydans.Protein J. 2009 Aug;28(6):263-72. doi: 10.1007/s10930-009-9191-2. Protein J. 2009. PMID: 19629658
-
Characterization of mannitol-2-dehydrogenase in Saccharina japonica: evidence for a new polyol-specific long-chain dehydrogenases/reductase.PLoS One. 2014 May 15;9(5):e97935. doi: 10.1371/journal.pone.0097935. eCollection 2014. PLoS One. 2014. PMID: 24830763 Free PMC article.
-
Inhibition kinetics and emodin cocrystal structure of a type II polyketide ketoreductase.Biochemistry. 2008 Feb 19;47(7):1837-47. doi: 10.1021/bi7016427. Epub 2008 Jan 19. Biochemistry. 2008. PMID: 18205400 Free PMC article.
References
-
- Aarnihunnas, J., K. Ronnholm, and A. Palava. 2002. The mannitol dehydrogenase gene (mdh) from Leuconostoc mesenteroides is distinct from other known bacterial mdh genes. Appl. Microbiol. Biotechnol. 59:665-671. - PubMed
-
- Armarego, W. L. 1979. Hydrogen transfer from 4-R and 4-S (4-3H) NADH in the reduction of d,l-cis-6,7-dimethyl-6,7 (8H) dihydropterin with dihydropteridine reductase from human liver and sheep liver. Biochem. Biophys. Res. Commun. 89:246-249. - PubMed
-
- Arnold, L. J., Jr., K. You, W. S. Allison, and N. O. Kaplan. 1976. Determination of the hydride transfer stereospecificity of nicotinamide adenine dinucleotide linked oxidoreductases by proton magnetic resonance. Biochemistry 15:4844-4849. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

