Colonization of Arabidopsis thaliana with Salmonella enterica and enterohemorrhagic Escherichia coli O157:H7 and competition by Enterobacter asburiae
- PMID: 12902287
- PMCID: PMC169118
- DOI: 10.1128/AEM.69.8.4915-4926.2003
Colonization of Arabidopsis thaliana with Salmonella enterica and enterohemorrhagic Escherichia coli O157:H7 and competition by Enterobacter asburiae
Abstract
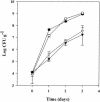
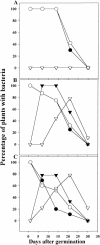
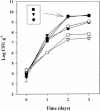
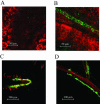
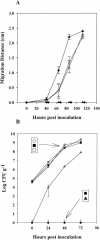
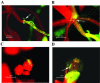
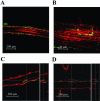
Enteric pathogens, such as Salmonella enterica and Escherichia coli O157:H7, have been shown to contaminate fresh produce. Under appropriate conditions, these bacteria will grow on and invade the plant tissue. We have developed Arabidopsis thaliana (thale cress) as a model system with the intention of studying plant responses to human pathogens. Under sterile conditions and at 100% humidity, S. enterica serovar Newport and E. coli O157:H7 grew to 10(9) CFU g(-1) on A. thaliana roots and to 2 x 10(7) CFU g(-1) on shoots. Furthermore, root inoculation led to contamination of the entire plant, indicating that the pathogens are capable of moving on or within the plant in the absence of competition. Inoculation with green fluorescent protein-labeled S. enterica and E. coli O157:H7 showed invasion of the roots at lateral root junctions. Movement was eliminated and invasion decreased when nonmotile mutants of S. enterica were used. Survival of S. enterica serovar Newport and E. coli O157:H7 on soil-grown plants declined as the plants matured, but both pathogens were detectable for at least 21 days. Survival of the pathogen was reduced in unautoclaved soil and amended soil, suggesting competition from indigenous epiphytes from the soil. Enterobacter asburiae was isolated from soil-grown A. thaliana and shown to be effective at suppressing epiphytic growth of both pathogens under gnotobiotic conditions. Seed and chaff harvested from contaminated plants were occasionally contaminated. The rate of recovery of S. enterica and E. coli O157:H7 from seed varied from undetectable to 19% of the seed pools tested, depending on the method of inoculation. Seed contamination by these pathogens was undetectable in the presence of the competitor, Enterobacter asburiae. Sampling of 74 pools of chaff indicated a strong correlation between contamination of the chaff and seed (P = 0.025). This suggested that contamination of the seed occurred directly from contaminated chaff or by invasion of the flower or silique. However, contaminated seeds were not sanitized by extensive washing and chlorine treatment, indicating that some of the bacteria reside in a protected niche on the seed surface or under the seed coat.
Figures
References
-
- Anonymous. 1997. Outbreaks of Escherichia coli O157:H7 infection and cryptosporidiosis associated with drinking unpasteurized apple cider—Connecticut and New York, October 1996. Morbid. Mortal. Wkly. Rep. 46:4-8. - PubMed
-
- Anonymous. 1996. Standardized molecular subtyping of foodborne bacterial pathogens by pulsed-field gel electrophoresis: a manual. National Center for Infectious Diseases, Centers for Disease Control and Prevention, Atlanta, Ga.
-
- Arora, D. K., A. B. Filonow, and J. L. Lockwood. 1983. Bacterial chemotaxis to fungal propagules in vitro and in soil. Can. J. Microbiol. 29:1104-1109.
-
- Bayot, R. G., and S. M. Ries. 1986. Role of motility in apple blossom infection by Erwinia amylovora and studies of fire blight control with attractant and repellent compounds. Phytopathology 76:441-445.
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

