The Ti plasmid of Agrobacterium tumefaciens harbors an attM-paralogous gene, aiiB, also encoding N-Acyl homoserine lactonase activity
- PMID: 12902298
- PMCID: PMC169067
- DOI: 10.1128/AEM.69.8.4989-4993.2003
The Ti plasmid of Agrobacterium tumefaciens harbors an attM-paralogous gene, aiiB, also encoding N-Acyl homoserine lactonase activity
Abstract
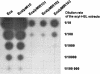
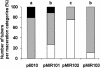
The Agrobacterium tumefaciens C58 genome contains three putative N-acyl homoserine lactone (acyl-HSL) hydrolases, which are closely related to the lactonase AiiA of Bacillus. When expressed in Escherichia coli, two of the putative acyl-HSL hydrolases, AttM and AiiB, conferred the ability to degrade acyl-HSLs on the host. In Erwinia strain 6276, the lactonases reduced the endogenous acyl-HSL level and the bacterial virulence in planta.
Figures



Similar articles
-
Different regulation and roles of lactonases AiiB and AttM in Agrobacterium tumefaciens C58.Mol Plant Microbe Interact. 2009 May;22(5):529-37. doi: 10.1094/MPMI-22-5-0529. Mol Plant Microbe Interact. 2009. PMID: 19348571
-
The assimilation of gamma-butyrolactone in Agrobacterium tumefaciens C58 interferes with the accumulation of the N-acyl-homoserine lactone signal.Mol Plant Microbe Interact. 2004 Sep;17(9):951-7. doi: 10.1094/MPMI.2004.17.9.951. Mol Plant Microbe Interact. 2004. PMID: 15384485
-
AhlD, an N-acylhomoserine lactonase in Arthrobacter sp., and predicted homologues in other bacteria.Microbiology (Reading). 2003 Jun;149(Pt 6):1541-1550. doi: 10.1099/mic.0.26269-0. Microbiology (Reading). 2003. PMID: 12777494
-
The Agrobacterium Ti Plasmids.Microbiol Spectr. 2014 Dec;2(6):10.1128/microbiolspec.PLAS-0010-2013. doi: 10.1128/microbiolspec.PLAS-0010-2013. Microbiol Spectr. 2014. PMID: 25593788 Free PMC article. Review.
-
A Brief History of Plasmids.EcoSal Plus. 2022 Dec 15;10(1):eESP00282021. doi: 10.1128/ecosalplus.esp-0028-2021. Epub 2022 Apr 4. EcoSal Plus. 2022. PMID: 35373578 Free PMC article. Review.
Cited by
-
A metagenomic study highlights phylogenetic proximity of quorum-quenching and xenobiotic-degrading amidases of the AS-family.PLoS One. 2013 Jun 7;8(6):e65473. doi: 10.1371/journal.pone.0065473. Print 2013. PLoS One. 2013. PMID: 23762380 Free PMC article.
-
The metabolic flux regulation of Klebsiella pneumoniae based on quorum sensing system.Sci Rep. 2016 Dec 7;6:38725. doi: 10.1038/srep38725. Sci Rep. 2016. PMID: 27924940 Free PMC article.
-
Structural and Biochemical Characterization of AidC, a Quorum-Quenching Lactonase with Atypical Selectivity.Biochemistry. 2015 Jul 21;54(28):4342-53. doi: 10.1021/acs.biochem.5b00499. Epub 2015 Jul 8. Biochemistry. 2015. PMID: 26115006 Free PMC article.
-
Synthesis of N-acyl homoserine lactone analogues reveals strong activators of SdiA, the Salmonella enterica serovar Typhimurium LuxR homologue.Appl Environ Microbiol. 2007 Jan;73(2):535-44. doi: 10.1128/AEM.01451-06. Epub 2006 Nov 3. Appl Environ Microbiol. 2007. PMID: 17085703 Free PMC article.
-
Messing with bacterial quorum sensing.Microbiol Mol Biol Rev. 2006 Dec;70(4):859-75. doi: 10.1128/MMBR.00002-06. Microbiol Mol Biol Rev. 2006. PMID: 17158701 Free PMC article. Review.
References
-
- Cha, C., P. Gao, Y. C. Chen, P. D. Shaw, and S. K. Farrand. 1998. Production of acyl-homoserine lactone quorum-sensing signals by gram-negative plant-associated bacteria. Mol. Plant-Microbe Interact. 11:1119-1129. - PubMed
-
- Daiyasu, H., K. Osaka, Y. Ishino, and H. Toh. 2001. Expansion of the zinc metallo-hydrolase family of the β-lactamase fold. FEBS Lett. 503:1-6. - PubMed
-
- Dong, Y. H., L. H. Wang, H. B. Zhang, X. F. Zhang, and L. H. Zhang. 2001. Quenching quorum-sensing-dependent bacterial infection by an N-acyl homoserine lactonase. Nature 411:813-817. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

