Mechanisms of ganglioside inhibition of APC function
- PMID: 12902465
- PMCID: PMC2849639
- DOI: 10.4049/jimmunol.171.4.1676
Mechanisms of ganglioside inhibition of APC function
Abstract
Gangliosides shed by tumor cells exert potent inhibitory effects on cellular immune responses. Here we have studied ganglioside inhibition of APC function. When human monocytes were preincubated in 50 micro M highly purified ganglioside G(D1a), pulsed with tetanus toxoid (TT), and washed, the expected Ag-induced proliferative response of autologous normal T cells added to these monocytes was inhibited by 81%. Strikingly, there was also almost complete (92%) and selective inhibition of the up-regulation of the monocyte costimulatory molecule CD80, while I-CAM-1, LFA-3, HLA-DR, and CD86 expression were unaffected. Purified LPS-stimulated monocytes that had been preincubated in G(D1a) likewise showed inhibition of CD80 up-regulation (59%) as well as down-regulation of CD40 (54%) and impaired release of IL-12 and TNF-alpha (reduced by 59 and 51%). G(D1a)-preincubated human dendritic cells (DC) were also affected. They had reduced constitutive expression of CD40 (33%) and CD80 (61%), but not CD86, and marked inhibition of release of IL-6 (72%), IL-12 (70%), and TNF-alpha (46%). Even when pulsed with TT, these ganglioside-preincubated DC remained deficient in costimulatory molecule expression and cytokine secretion and were unable to induce a normal T cell proliferative response to TT. Finally, significant inhibition of nuclear localization of NF-kappaB proteins in activated DC suggests that disruption of NF-kappaB activation may be one mechanism contributing to ganglioside interference with APC expression of costimulatory molecules and cytokine secretion, which, in turn, may diminish antitumor immune responses.
Figures




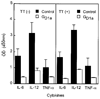
 , results obtained by incubating GD1a-treated and control DC as described above and then conincubating them for an additional 24 h with autologous T cells before determining costimulatory molecule expression.
, results obtained by incubating GD1a-treated and control DC as described above and then conincubating them for an additional 24 h with autologous T cells before determining costimulatory molecule expression.

Similar articles
-
Ganglioside GD1a impedes lipopolysaccharide-induced maturation of human dendritic cells.Cell Immunol. 2002 Dec;220(2):125-33. doi: 10.1016/s0008-8749(03)00004-2. Cell Immunol. 2002. PMID: 12657247
-
Gangliosides block antigen presentation by human monocytes.Biochim Biophys Acta. 1996 Sep 27;1303(2):161-8. doi: 10.1016/0005-2760(96)00091-4. Biochim Biophys Acta. 1996. PMID: 8856046
-
Regulation of human dendritic cells by a novel specific nuclear factor-kappaB inhibitor, dehydroxymethylepoxyquinomicin.Hum Immunol. 2010 Aug;71(8):763-70. doi: 10.1016/j.humimm.2010.05.009. Epub 2010 May 24. Hum Immunol. 2010. PMID: 20573582
-
Glucocorticoids inhibit bioactive IL-12p70 production by in vitro-generated human dendritic cells without affecting their T cell stimulatory potential.J Immunol. 1998 Nov 15;161(10):5245-51. J Immunol. 1998. PMID: 9820496
-
The sesquiterpene lactone parthenolide inhibits LPS- but not TNF-alpha-induced maturation of human monocyte-derived dendritic cells by inhibition of the p38 mitogen-activated protein kinase pathway.J Allergy Clin Immunol. 2002 Aug;110(2):269-76. doi: 10.1067/mai.2002.126381. J Allergy Clin Immunol. 2002. PMID: 12170268
Cited by
-
Aberrant Glycosylation as Immune Therapeutic Targets for Solid Tumors.Cancers (Basel). 2023 Jul 8;15(14):3536. doi: 10.3390/cancers15143536. Cancers (Basel). 2023. PMID: 37509200 Free PMC article. Review.
-
Role of tumour-associated N-glycolylated variant of GM3 ganglioside in cancer progression: effect over CD4 expression on T cells.Cancer Immunol Immunother. 2006 Apr;55(4):443-50. doi: 10.1007/s00262-005-0041-6. Epub 2005 Oct 6. Cancer Immunol Immunother. 2006. PMID: 16208470 Free PMC article.
-
The cancer glycocode as a family of diagnostic biomarkers, exemplified by tumor-associated gangliosides.Front Oncol. 2023 Oct 26;13:1261090. doi: 10.3389/fonc.2023.1261090. eCollection 2023. Front Oncol. 2023. PMID: 37954075 Free PMC article. Review.
-
Reprogramming the tumor microenvironment: tumor-induced immunosuppressive factors paralyze T cells.Oncoimmunology. 2015 Apr 1;4(7):e1016700. doi: 10.1080/2162402X.2015.1016700. eCollection 2015 Jul. Oncoimmunology. 2015. PMID: 26140242 Free PMC article. Review.
-
Induction of lysosomal and plasma membrane-bound sialidases in human T-cells via T-cell receptor.Biochem J. 2004 Jun 1;380(Pt 2):425-33. doi: 10.1042/BJ20031896. Biochem J. 2004. PMID: 14992689 Free PMC article.
References
-
- Caldwell SA, Heitger A, Taylor B, Ladisch S. Gangliosides inhibit antigen-presenting cell costimulatory activity of monocytes and dendritic cells. Proc. Am. Assoc. Cancer Res. 2000;41:115.
-
- Elgert KD, Alleva DG, Mullins DW. Tumor-induced immune dysfunction: the macrophage connection. J. Leukocyte Biol. 1998;64:275. - PubMed
-
- Miescher S, Whiteside TL, Carrel S, von Fliedner V. Functional properties of tumor-infiltrating and blood lymphocytes in patients with solid tumors: effects of tumor cells and their supernatants on proliferative responses of lymphocytes. J. Immunol. 1986;136:1899. - PubMed
-
- Matulonis U, Dosiou C, Freeman G, Lamont C, Mauch P, Nadler LM, Griffin JD. B7-1 is superior to B7-2 costimulation in the induction and maintenance of T cell-mediated antileukemia immunity: further evidence that B7-1 and B7-2 are functionally distinct. J. Immunol. 1996;156:1126. - PubMed
-
- Chaux P, Favre N, Martin M, Martin F. Tumor-infiltrating dendritic cells are defective in their antigen-presenting function and inducible B7 expression in rats. Int. J. Cancer. 1997;72:619. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

