Intracellular domain of brain endothelial intercellular adhesion molecule-1 is essential for T lymphocyte-mediated signaling and migration
- PMID: 12902516
- PMCID: PMC3831576
- DOI: 10.4049/jimmunol.171.4.2099
Intracellular domain of brain endothelial intercellular adhesion molecule-1 is essential for T lymphocyte-mediated signaling and migration
Abstract
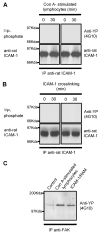
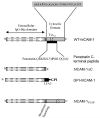
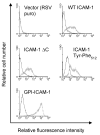
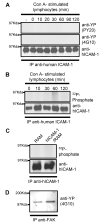
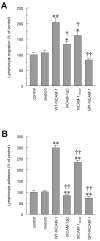
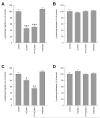
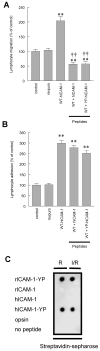
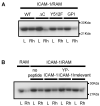
To examine the role of the ICAM-1 C-terminal domain in transendothelial T lymphocyte migration and ICAM-1-mediated signal transduction, mutant human (h)ICAM-1 molecules were expressed in rat brain microvascular endothelial cells. The expression of wild-type hICAM-1 resulted in a significant increase over basal levels in both adhesion and transendothelial migration of T lymphocytes. Endothelial cells (EC) expressing ICAM-1 in which the tyrosine residue at codon 512 was substituted with phenylalanine (hICAM-1(Y512F)) also exhibited increased lymphocyte migration, albeit less than that with wild-type hICAM-1. Conversely, the expression of truncated hICAM-1 proteins, in which either the intracellular domain was deleted (hICAM-1DeltaC) or both the intracellular and transmembrane domains were deleted through construction of a GPI anchor (GPI-hICAM-1), did not result in an increase in lymphocyte adhesion, and their ability to increase transendothelial migration was attenuated. Truncated hICAM-1 proteins were also unable to induce ICAM-1-mediated Rho GTPase activation. EC treated with cell-permeant penetratin-ICAM-1 peptides comprising human or rat ICAM-1 intracellular domain sequences inhibited transendothelial lymphocyte migration, but not adhesion. Peptides containing a phosphotyrosine residue were equipotent in inhibiting lymphocyte migration. These data demonstrate that the intracellular domain of ICAM-1 is essential for transendothelial migration of lymphocytes, and that peptidomimetics of the ICAM-1 intracellular domain can also inhibit this process. Such competitive inhibition of transendothelial lymphocyte migration in the absence of an affect on adhesion further implicates ICAM-1-mediated signaling events in the facilitation of T lymphocyte migration across brain EC. Thus, agents that mimic the ICAM-1 intracellular domain may be attractive targets for novel anti-inflammatory therapeutics.
Figures

References
-
- Male D, Pryce G, Hughes C, Lantos P. Lymphocyte migration into brain modeled in vitro: control by lymphocyte activation, cytokines, and antigen. Cell. Immunol. 1990;127:1. - PubMed
-
- Pryce G, Male DK, Campbell I, Greenwood J. Factors controlling T-cell migration across rat cerebral endothelium in vitro. J. Neuroimmunol. 1997;75:84. - PubMed
-
- Etienne S, Adamson P, Greenwood J, Strosberg AD, Cazaubon S, Couraud P-O. Rho-dependent signaling pathways coupled to ICAM-1 in microvascular brain endothelial cells. J. Immunol. 1998;161:5755. - PubMed
-
- Adamson P, Etienne S, Couraud P-O, Calder V, Greenwood J. Lymphocyte migration through brain endothelial cell monolayers involves signalling through endothelial ICAM-1 via a Rho-dependent pathway. J. Immunol. 1999;162:2964. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

