The origin and liver repopulating capacity of murine oval cells
- PMID: 12902545
- PMCID: PMC304102
- DOI: 10.1073/pnas.1734199100
The origin and liver repopulating capacity of murine oval cells
Abstract
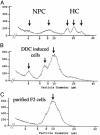
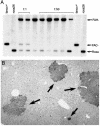
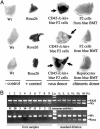
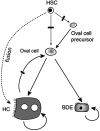
The appearance of bipotential oval cells in chronic liver injury suggests the existence of hepatocyte progenitor/stem cells. To study the origin and properties of this cell population, oval cell proliferation was induced in adult mouse liver by 3,5-diethoxycarbonyl-1,4-dihydrocollidine (DDC) and a method for their isolation was developed. Transplantation into fumarylacetoacetate hydrolase (Fah) deficient mice was used to determine their capacity for liver repopulation. In competitive repopulation experiments, hepatic oval cells were at least as efficient as mature hepatocytes in repopulating the liver. In mice with chimeric livers, the oval cells were not derived from hepatocytes but from liver nonparenchymal cells. This finding supports a model in which intrahepatic progenitors differentiate into hepatocytes irreversibly. To determine whether oval cells originated from stem cells residing in the bone marrow, bone marrow transplanted wild-type mice were treated with DDC for 8 months and oval cells were then serially transferred into Fah mutants. The liver repopulating cells in these secondary transplant recipients lacked the genetic markers of the original bone marrow donor. We conclude that hepatic oval cells do not originate in bone marrow but in the liver itself, and that they have valuable properties for therapeutic liver repopulation.
Figures



Similar articles
-
Therapeutic liver reconstitution with murine cells isolated long after death.Gastroenterology. 2010 Sep;139(3):1019-29. doi: 10.1053/j.gastro.2010.05.082. Epub 2010 Jun 2. Gastroenterology. 2010. PMID: 20621682 Free PMC article.
-
Disparate cellular basis of improved liver repair in beta-catenin-overexpressing mice after long-term exposure to 3,5-diethoxycarbonyl-1,4-dihydrocollidine.Am J Pathol. 2010 Oct;177(4):1812-22. doi: 10.2353/ajpath.2010.100173. Epub 2010 Sep 2. Am J Pathol. 2010. PMID: 20813968 Free PMC article.
-
Purified hematopoietic stem cells can differentiate into hepatocytes in vivo.Nat Med. 2000 Nov;6(11):1229-34. doi: 10.1038/81326. Nat Med. 2000. PMID: 11062533
-
Fah Knockout Animals as Models for Therapeutic Liver Repopulation.Adv Exp Med Biol. 2017;959:215-230. doi: 10.1007/978-3-319-55780-9_20. Adv Exp Med Biol. 2017. PMID: 28755199 Review.
-
Hepatic oval (stem) cell expression of endothelial differentiation gene receptors for lysophosphatidic acid in mouse chronic liver injury.J Hematother Stem Cell Res. 2002 Aug;11(4):643-9. doi: 10.1089/15258160260194785. J Hematother Stem Cell Res. 2002. PMID: 12201952 Review.
Cited by
-
Potentials of regenerative medicine for liver disease.Surg Today. 2009;39(12):1019-25. doi: 10.1007/s00595-009-4056-z. Epub 2009 Dec 8. Surg Today. 2009. PMID: 19997795 Review.
-
Cell therapy for the diseased liver: from stem cell biology to novel models for hepatotropic human pathogens.Dis Model Mech. 2008 Sep-Oct;1(2-3):113-30. doi: 10.1242/dmm.000463. Dis Model Mech. 2008. PMID: 19048074 Free PMC article.
-
Clonal tracing of Sox9+ liver progenitors in mouse oval cell injury.Hepatology. 2014 Jul;60(1):278-89. doi: 10.1002/hep.27084. Epub 2014 May 28. Hepatology. 2014. PMID: 24700457 Free PMC article.
-
Mammalian Target of Rapamycin Complex 2 Signaling Is Required for Liver Regeneration in a Cholestatic Liver Injury Murine Model.Am J Pathol. 2020 Jul;190(7):1414-1426. doi: 10.1016/j.ajpath.2020.03.010. Epub 2020 Apr 7. Am J Pathol. 2020. PMID: 32275903 Free PMC article.
-
Bipotential adult liver progenitors are derived from chronically injured mature hepatocytes.Cell Stem Cell. 2014 Nov 6;15(5):605-18. doi: 10.1016/j.stem.2014.09.008. Epub 2014 Oct 9. Cell Stem Cell. 2014. PMID: 25312494 Free PMC article.
References
-
- Michalopoulos, G. K. & DeFrances, M. C. (1997) Science 276, 60-66. - PubMed
-
- Grompe, M. & Finegold, M. (2001) in Stem Cell Biology, eds. Marshak, D. R., Gardner, R. L. & Gottlieb, D. (Cold Spring Harbor Lab. Press, Plainview, NY), pp. 455-497.
-
- Fausto, N. & Campbell, J. S. (2003) Mech. Dev. 120, 117-130. - PubMed
-
- Shinozuka, H., Lombardi, B., Sell, S. & Iammarino, R. M. (1978) Cancer Res. 38, 1092-1098. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

