Estrogen in the adult male reproductive tract: a review
- PMID: 12904263
- PMCID: PMC179885
- DOI: 10.1186/1477-7827-1-52
Estrogen in the adult male reproductive tract: a review
Abstract
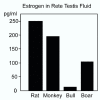
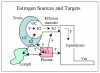
Testosterone and estrogen are no longer considered male only and female only hormones. Both hormones are important in both sexes. It was known as early as the 1930's that developmental exposure to a high dose of estrogen causes malformation of the male reproductive tract, but the early formative years of reproductive biology as a discipline did not recognize the importance of estrogen in regulating the normal function of the adult male reproductive tract. In the adult testis, estrogen is synthesized by Leydig cells and the germ cells, producing a relatively high concentration in rete testis fluid. Estrogen receptors are present in the testis, efferent ductules and epididymis of most species. However, estrogen receptor-alpha is reported absent in the testis of a few species, including man. Estrogen receptors are abundant in the efferent ductule epithelium, where their primary function is to regulate the expression of proteins involved in fluid reabsorption. Disruption of the alpha-receptor, either in the knockout (alphaERKO) or by treatment with a pure antiestrogen, results in dilution of cauda epididymal sperm, disruption of sperm morphology, inhibition of sodium transport and subsequent water reabsorption, increased secretion of Cl-, and eventual decreased fertility. In addition to this primary regulation of luminal fluid and ion transport, estrogen is also responsible for maintaining a differentiated epithelial morphology. Thus, we conclude that estrogen or its alpha-receptor is an absolute necessity for fertility in the male.
Figures




References
-
- Wolff E, Ginglinger A. Sur la transformation des Poulets males en intersexues par injection d'hormone femelle (folliculine) aux embryons. Archs Anat Histol Embryol. 1935;20:219–278.
-
- Weniger JP. Aromatase activity in fetal gonads of mammals. J Dev Physiol. 1990;14:303–306. - PubMed
-
- Burrows H. Pathological conditions induced by oestrogenic compounds in the coagulating gland and prostate of the mouse. Am J Cancer. 1935;23:490–512.
-
- Greene RR, Burrill MW, Ivy AC. Experimental intersexuality. Am J Anat. 1940;67:305–345.
-
- Arai Y, Mori T, Suzuki Y, Bern H. Long-term effects of perinatal exposure to sex steroids and diethylstilbestrol on the reproductive system of male mammals. Int Rev Cytol. 1983;84:235–265. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

