Patterned vision causes CRE-mediated gene expression in the visual cortex through PKA and ERK
- PMID: 12904462
- PMCID: PMC6740662
- DOI: 10.1523/JNEUROSCI.23-18-07012.2003
Patterned vision causes CRE-mediated gene expression in the visual cortex through PKA and ERK
Abstract
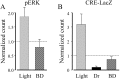
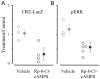
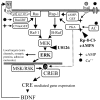
Normal visual experience during postnatal development is necessary for the maturation of visual cortical circuits and acts through molecular mechanisms that are still poorly understood. Recently, it has been shown that ERK (extracellular signal-regulated kinase) 1/2, protein kinase A (PKA), and CREB (cAMP response element-binding protein) are crucial factors for experience-dependent development of the visual cortex, but very little is known about the role of visual experience in their activation. Here, we show that visual stimulation after a brief period of dark rearing caused a transient ERK activation in the visual cortex. Visually induced ERK activation occurred primarily in excitatory neurons of layers II-III and VI and was prevented by binocular lid suture. ERK phosphorylation was strongly reduced by cortical infusion with the cAMP-PKA inhibitor Rp-8-Cl-cAMPS, thus establishing a link between PKA and ERK activation. To analyze the downstream consequences of ERK and PKA signaling, we studied the action of visual stimulation on transcription of genes controlled by CREB in transgenic mice carrying the LacZ reporter gene under the control of the CRE (cAMP response element) promoter. Visual stimulation triggered a prolonged episode of CRE-mediated gene expression in the visual cortex that was suppressed by infusion with the ERK inhibitor U0126. Cortical administration of Rp-8-Cl-cAMPS attenuated the experience-dependent activation of CRE-mediated gene transcription. These results show that ERK phosphorylation in visual cortical neurons represents a molecular readout of patterned visual stimuli and that visual activation of ERK involves the cAMP-PKA system. Finally, because CRE-mediated gene expression was totally dependent on ERK activation, we suggest that PKA action on CRE-mediated gene expression is mediated by ERK.
Figures
References
-
- Azouz R, Gray CM, Nowak LG, McCormick DA ( 1997) Physiological properties of inhibitory interneurons in cat striate cortex. Cereb Cortex 7: 534-545. - PubMed
-
- Bading H, Greenberg ME ( 1991) Stimulation of protein tyrosine phosphorylation by NMDA receptor activation. Science 253: 912-914. - PubMed
-
- Beaver CJ, Ji Q, Fischer QS, Daw NW ( 2001) Cyclic AMP-dependent protein kinase mediates ocular dominance shifts in cat visual cortex. Nat Neurosci 4: 159-163. - PubMed
-
- Berkeley JL, Decker MJ, Levey AI ( 2002) The role of muscarinic acetylcholine receptor-mediated activation of extracellular signal-regulated kinase 1/2 in pilocarpine-induced seizures. J Neurochem 82: 192-201. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous
