Selective antagonism of GluR5 kainate-receptor-mediated synaptic currents by topiramate in rat basolateral amygdala neurons
- PMID: 12904467
- PMCID: PMC6740668
- DOI: 10.1523/JNEUROSCI.23-18-07069.2003
Selective antagonism of GluR5 kainate-receptor-mediated synaptic currents by topiramate in rat basolateral amygdala neurons
Abstract
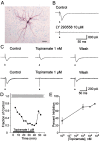
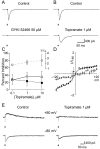
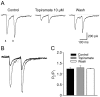
Topiramate is a widely used antiepileptic agent whose mechanism of action is poorly understood. The drug has been reported to interact with various ion channel types, including AMPA/kainate receptors. In whole-cell voltage-clamp recordings from principal neurons of the rat basolateral amygdala, topiramate at low concentrations (IC50, approximately 0.5 microm) selectively inhibited pharmacologically isolated excitatory synaptic currents mediated by kainate receptors containing the GluR5 subunit. Topiramate also partially depressed predominantly AMPA-receptor-mediated EPSCs, but with lower efficacy. Topiramate did not alter the degree of facilitation in paired-pulse experiments, and it reduced the amplitude of miniature EPSCs without affecting their frequency, demonstrating that the block of synaptic responses occurs postsynaptically. Inhibition of GluR5 kainate receptors could represent a key mechanism underlying the anticonvulsant activity of topiramate. Moreover, these results support the concept that GluR5 kainate receptors represent a novel target for antiepileptic drug development.
Figures

References
-
- Bleakman D, Lodge D ( 1998) Neuropharmacology of AMPA and kainate receptors. Neuropharmacology 37: 1187-1204. - PubMed
-
- Bleakman D, Gates MR, Ogden AM, Mackowiak M ( 2002) Kainate receptor agonists, antagonists and allosteric modulators. Curr Pharm Des 8: 873- 885. - PubMed
-
- Castillo PE, Malenka RC, Nicoll RA ( 1997) Kainate receptors mediate a slow postsynaptic current in hippocampal CA3 neurons. Nature 388: 182-186. - PubMed
-
- Chong MS, Libretto SE ( 2003) The rationale and use of topiramate for treating neuropathic pain. Clin J Pain 19: 59-68. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
