Medical prescription of heroin to treatment resistant heroin addicts: two randomised controlled trials
- PMID: 12907482
- PMCID: PMC169643
- DOI: 10.1136/bmj.327.7410.310
Medical prescription of heroin to treatment resistant heroin addicts: two randomised controlled trials
Erratum in
- BMJ. 2003 Sep 27;3217(7417):724
Abstract
Objective: To determine whether supervised medical prescription of heroin can successfully treat addicts who do not sufficiently benefit from methadone maintenance treatment.
Design: Two open label randomised controlled trials.
Setting: Methadone maintenance programmes in six cities in the Netherlands.
Participants: 549 heroin addicts.
Interventions: Inhalable heroin (n = 375) or injectable heroin (n = 174) prescribed over 12 months. Heroin (maximum 1000 mg per day) plus methadone (maximum 150 mg per day) compared with methadone alone (maximum 150 mg per day). Psychosocial treatment was offered throughout.
Main outcome measures: Dichotomous, multidomain response index, including validated indicators of physical health, mental status, and social functioning.
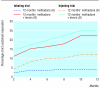
Results: Adherence was excellent with 12 month outcome data available for 94% of the randomised participants. With intention to treat analysis, 12 month treatment with heroin plus methadone was significantly more effective than treatment with methadone alone in the trial of inhalable heroin (response rate 49.7% v 26.9%; difference 22.8%, 95% confidence interval 11.0% to 34.6%) and in the trial of injectable heroin (55.5% v 31.2%; difference 24.3%, 9.6% to 39.0%). Discontinuation of the coprescribed heroin resulted in a rapid deterioration in 82% (94/115) of those who responded to the coprescribed heroin. The incidence of serious adverse events was similar across treatment conditions.
Conclusions: Supervised coprescription of heroin is feasible, more effective, and probably as safe as methadone alone in reducing the many physical, mental, and social problems of treatment resistant heroin addicts.
Figures

Comment in
-
Prescription of heroin to treatment resistant heroin addicts: Dutch heroin trials show retention is better with methadone alone.BMJ. 2004 Jan 24;328(7433):228-9; author reply 229. doi: 10.1136/bmj.328.7433.228-c. BMJ. 2004. PMID: 14739202 Free PMC article. No abstract available.
-
Prescription of heroin to treatment resistant heroin addicts: double blinding is not possible.BMJ. 2004 Jan 24;328(7433):228; author reply 229. doi: 10.1136/bmj.328.7433.228. BMJ. 2004. PMID: 14739203 Free PMC article. No abstract available.
-
Prescription of heroin to treatment resistant heroin addicts: treatment needs to be multifaceted.BMJ. 2004 Jan 24;328(7433):228; author reply 229. doi: 10.1136/bmj.328.7433.228-b. BMJ. 2004. PMID: 14739204 Free PMC article. No abstract available.
-
Prescription of heroin to treatment resistant heroin addicts: replacement therapies need to be tested on a level playing field.BMJ. 2004 Jan 24;328(7433):228; author reply 229. doi: 10.1136/bmj.328.7433.228-a. BMJ. 2004. PMID: 14739205 Free PMC article. No abstract available.
-
Prescription of heroin to treatment resistant heroin addicts: heroin handouts are flawed policy.BMJ. 2004 Jan 24;328(7433):229; author reply 229. doi: 10.1136/bmj.328.7433.229. BMJ. 2004. PMID: 14739208 Free PMC article. No abstract available.
-
Supervised co-prescription of heroin to treatment-resistant heroin addicts is more effective than treatment with methadone alone.Evid Based Ment Health. 2004 Feb;7(1):23. doi: 10.1136/ebmh.7.1.23. Evid Based Ment Health. 2004. PMID: 14769667 No abstract available.
References
-
- Van den Brink W, Hendriks VM, Van Ree J. Medical co-prescription of heroin to chronic, treatment-resistant methadone patients in the Netherlands. J Drug Issues 1999;29: 587-608.
-
- Nationale Drug Monitor (NDM). Jaarbericht NDM 2001. Utrecht: Bureau NDM, 2001.
-
- Driessen FMHM. Methadonverstrekking in Nederland. Utrecht: Bureau Driessen, 1990.
-
- Driessen FMHM. Methadoncliënten in Nederland. Utrecht: Bureau Driessen, 1992.
-
- Driessen FMHM, Völker BGM, Kregting J, Van der Lelij B. De ontwikkeling van de situatie van methadoncliënten gedurende twee jaar. Utrecht: Bureau Driessen, 1999.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
