Chronic hepatitis associated with GB virus B persistence in a tamarin after intrahepatic inoculation of synthetic viral RNA
- PMID: 12907703
- PMCID: PMC187902
- DOI: 10.1073/pnas.1731505100
Chronic hepatitis associated with GB virus B persistence in a tamarin after intrahepatic inoculation of synthetic viral RNA
Abstract
Progress in understanding the pathogenesis of hepatitis C virus (HCV) has been slowed by the absence of tractable small animal models. Whereas GB virus B (GBV-B, an unclassified flavivirus) shares a phylogenetic relationship and several biologic attributes with HCV, including hepatotropism, it is not known to cause persistent infection, a hallmark of HCV. Here, we document persistent GBV-B infection in one of two healthy tamarins (Saguinus oedipus) inoculated intrahepatically with infectious synthetic RNA. High-titer viremia (108 to 109 genome equivalents per ml) and transiently elevated serum alanine transaminase activities were present from weeks 4 to 12 postinoculation in both animals. However, whereas GBV-B was eliminated from one animal by 20 weeks, the second animal remained viremic (103 to 107 genome equivalents per ml) for >2 years, with alanine transaminase levels becoming elevated again before spontaneous resolution of the infection. A liver biopsy taken late in the course of infection demonstrated hepatitis with periportal mononuclear infiltrates, hepatocellular microvesicular changes, cytoplasmic lipid droplets, and disordered mitochondrial ultrastructure, findings remarkably similar to chronic hepatitis C. GBV-B-infected hepatocytes contained numerous small vesicular membranous structures resembling those associated with expression of HCV nonstructural proteins, and sequencing of GBV-B RNA demonstrated a rate of molecular evolution comparable to that of HCV. We conclude that GBV-B is capable of establishing persistent infections in healthy tamarins, a feature that substantially enhances its value as a model for HCV. Mitochondrial structural changes and altered lipid metabolism leading to steatosis are conserved features of the pathogenesis of chronic hepatitis caused by these genetically distinct flaviviruses.
Figures



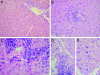

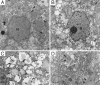
 ,
mitochondria, with ill-defined cristae in the infected tissue. →, lipid
droplets with electron-dense surface deposits. ▸, clusters of small
smooth-surface vesicles. N, nucleus. (Bar, 1 μm.)
,
mitochondria, with ill-defined cristae in the infected tissue. →, lipid
droplets with electron-dense surface deposits. ▸, clusters of small
smooth-surface vesicles. N, nucleus. (Bar, 1 μm.)
Similar articles
-
Immunity against the GBV-B hepatitis virus in tamarins can prevent productive infection following rechallenge and is long-lived.J Med Virol. 2008 Jan;80(1):87-94. doi: 10.1002/jmv.21013. J Med Virol. 2008. PMID: 18041000
-
Infection of Common Marmosets with GB Virus B Chimeric Virus Encoding the Major Nonstructural Proteins NS2 to NS4A of Hepatitis C Virus.J Virol. 2016 Aug 26;90(18):8198-211. doi: 10.1128/JVI.02653-15. Print 2016 Sep 15. J Virol. 2016. PMID: 27384651 Free PMC article.
-
In vivo analysis of the 3' untranslated region of GB virus B after in vitro mutagenesis of an infectious cDNA clone: persistent infection in a transfected tamarin.J Virol. 2004 Sep;78(17):9389-99. doi: 10.1128/JVI.78.17.9389-9399.2004. J Virol. 2004. PMID: 15308733 Free PMC article.
-
GB virus B as a model for hepatitis C virus.ILAR J. 2001;42(2):152-60. doi: 10.1093/ilar.42.2.152. ILAR J. 2001. PMID: 11406717 Review.
-
The GB hepatitis viruses.J Viral Hepat. 1995;2(5):221-6. doi: 10.1111/j.1365-2893.1995.tb00033.x. J Viral Hepat. 1995. PMID: 8745313 Review.
Cited by
-
Clinical and molecular aspects of human pegiviruses in the interaction host and infectious agent.Virol J. 2022 Mar 9;19(1):41. doi: 10.1186/s12985-022-01769-3. Virol J. 2022. PMID: 35264187 Free PMC article. Review.
-
The marmoset model of GB virus B infections: adaptation to host phenotypic variation.J Virol. 2009 Jun;83(11):5806-14. doi: 10.1128/JVI.00033-09. Epub 2009 Mar 11. J Virol. 2009. PMID: 19279089 Free PMC article.
-
Antiviral activity and host gene induction by tamarin and marmoset interferon-alpha and interferon-gamma in the GBV-B primary hepatocyte culture model.Virology. 2009 Aug 1;390(2):186-96. doi: 10.1016/j.virol.2009.05.005. Epub 2009 Jun 7. Virology. 2009. PMID: 19501869 Free PMC article.
-
Characterization of natural killer cells in tamarins: a technical basis for studies of innate immunity.Front Microbiol. 2010 Dec 8;1:128. doi: 10.3389/fmicb.2010.00128. eCollection 2010. Front Microbiol. 2010. PMID: 21713119 Free PMC article.
-
GB virus B disrupts RIG-I signaling by NS3/4A-mediated cleavage of the adaptor protein MAVS.J Virol. 2007 Jan;81(2):964-76. doi: 10.1128/JVI.02076-06. Epub 2006 Nov 8. J Virol. 2007. PMID: 17093192 Free PMC article.
References
-
- Seeff, L. B. & Hoofnagle, J. H. (2002) Hepatology 36, S1–S2. - PubMed
-
- Zein, C. O. & Zein, N. N. (2002) Microbes Infect. 4, 1237–1246. - PubMed
-
- Abe, K., Inchauspe, G., Shikata, T. & Prince, A. M. (1992) Hepatology 15, 690–695. - PubMed
-
- Farci, P., London, W. T., Wong, D. C., Dawson, G. J., Vallari, D. S., Engle, R. & Purcell, R. H. (1992) J. Infect. Dis. 165, 1006–1011. - PubMed
-
- Kolykhalov, A. A., Agapov, E. V., Blight, K. J., Mihalik, K., Feinstone, S. M. & Rice, C. M. (1997) Science 277, 570–574. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

