Insertion of the T3 DNA polymerase thioredoxin binding domain enhances the processivity and fidelity of Taq DNA polymerase
- PMID: 12907710
- PMCID: PMC169918
- DOI: 10.1093/nar/gkg667
Insertion of the T3 DNA polymerase thioredoxin binding domain enhances the processivity and fidelity of Taq DNA polymerase
Abstract
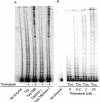
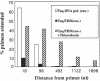
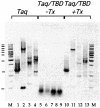
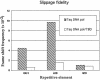
Insertion of the T3 DNA polymerase thioredoxin binding domain (TBD) into the distantly related thermostable Taq DNA polymerase at an analogous position in the thumb domain, converts the Taq DNA polymerase from a low processive to a highly processive enzyme. Processivity is dependent on the presence of thioredoxin. The enhancement in processivity is 20-50-fold when compared with the wild-type Taq DNA polymerase or to the recombinant polymerase in the absence of thioredoxin. The recombinant Taq DNA pol/TBD is thermostable, PCR competent and able to copy repetitive deoxynucleotide sequences six to seven times more faithfully than Taq DNA polymerase and makes 2-3-fold fewer AT-->GC transition mutations.
Figures


References
-
- Sia E.A., Jinks-Robertson,S. and Petes,T.D. (1997) Genetic control of microsatellite stability. Mutat. Res., 383, 61–70. - PubMed
-
- Kunkel T.A. and Alexander,P.S. (1986) The base substitution fidelity of eucaryotic DNA polymerases. Mispairing frequencies, site preferences, insertion preferences and base substitution by dislocation. J. Biol. Chem., 261, 160–166. - PubMed
-
- Himawan J.S. and Richardson,C.C. (1996) Amino acid residues critical for the interaction between bacteriophage T7 DNA polymerase and Escherichia coli thioredoxin. J. Biol. Chem., 271, 19999–20008. - PubMed
-
- Tabor S., Huber,H.E. and Richardson,C.C. (1987) Escherichia coli thioredoxin confers processivity on the DNA polymerase activity of the gene 5 protein of bacteriophage T7. J. Biol. Chem., 262, 16212–16223. - PubMed
-
- Huber H.E., Tabor,S. and Richardson,C.C. (1987) Escherichia coli thioredoxin stabilizes complexes of bacteriophage T7 DNA polymerase and primed templates. J. Biol. Chem., 262, 16224–16232. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

