GABAA and glycine receptors in regulation of intercostal and abdominal expiratory activity in vitro in neonatal rat
- PMID: 12909685
- PMCID: PMC2343214
- DOI: 10.1113/jphysiol.2003.042689
GABAA and glycine receptors in regulation of intercostal and abdominal expiratory activity in vitro in neonatal rat
Abstract
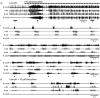
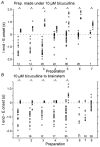
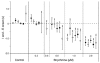
The roles played by GABAA and glycine receptors in inspiratory-expiratory motor co-ordination and in tonic inhibitory regulation of expiratory motor activity were studied using brainstem-spinal cord (-rib) preparations from neonatal rats. Inspiratory activity was recorded from the C4 ventral root. Expiratory activity in internal intercostal muscle, internal oblique muscle or T13 ventral root was evoked by a decrease in perfusate pH from 7.4 to 7.1 (i.e. from normal to low pH conditions) and was limited to the first part of the expiratory phase. Under low pH conditions, bath application of 10 microM bicuculline, a GABAA receptor antagonist, caused the inspiratory burst to overlap the expiratory burst in 2/7 preparations. Overlapping of the expiratory burst with the inspiratory burst was observed in 7/7 preparations made under 10 microM bicuculline. Furthermore, such preparations exhibited expiratory bursts under bicuculline-containing normal pH conditions. Local application of 10 microM bicuculline to the brainstem under normal pH conditions evoked expiratory bursts, some of which overlapped the inspiratory bursts. Picrotoxin, another antagonist of the GABAA receptor, had similar effects. Under normal pH conditions, application of strychnine (0.2- 2.0 microM; a glycine receptor antagonist) to the brainstem did not evoke expiratory bursts. On subsequent application of strychnine-containing low pH solution, expiratory bursts were evoked and some (0.5 microM) or all (2.0 microM) of these overlapped the inspiratory burst. Simultaneous application of picrotoxin and strychnine to the brainstem evoked expiratory bursts that overlapped the inspiratory bursts and a subsequent decrease in perfusate pH to 7.1 increased the frequency of the respiratory rhythm. It was a characteristic finding that the duration of the expiratory burst exceeded that of the inspiratory burst under control low pH conditions. This remained true during concurrent blockade of GABAA and glycine receptors. The results suggest that in the in vitro preparation from neonatal rats: (1) GABAA and glycine receptors within the brainstem play important roles in the co-ordination between inspiratory and expiratory motor activity, (2) tonic inhibition via GABAA receptors, but not glycine receptors, plays a role in the regulation of expiratory motor activity and (3) inspiratory and expiratory burst termination is independent of both GABAA and glycine receptors.
Figures






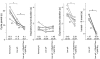
References
-
- Arata A, Onimaru H, Homma I. Possible synaptic connections of expiratory neurons in the medulla of newborn rat in vitro. Neuroreport. 1998;9:743–746. - PubMed
-
- Bajic J, Zuperku EJ, Tonkovic-Capin M, Hopp FA. Expiratory bulbospinal neurons of dog. I. Control of discharge patterns by pulmonary stretch receptors. Am J Physiol. 1992;262:R1075–1086. - PubMed
-
- Brockhaus J, Ballanyi K. Synaptic inhibition in the isolated respiratory network of neonatal rats. Eur J Neurosci. 1998;10:3823–3839. - PubMed
-
- Büsselberg D, Bischoff AM, Paton JFR, Richter DW. Reorganisation of respiratory network activity after loss of glycinergic inhibition. Pfugers Arch. 2001;441:444–449. - PubMed
-
- Dogas Z, Krolo M, Stuth EA, Tonkovic-Capin M, Hopp FA, McCrimmon DR, Zuperku EJ. Differential effects of GABAA receptor antagonists in the control of respiratory neuronal discharge patterns. J Neurophysiol. 1998;80:2368–2377. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

