In vivo regeneration of murine prostate from dissociated cell populations of postnatal epithelia and urogenital sinus mesenchyme
- PMID: 12909713
- PMCID: PMC304104
- DOI: 10.1073/pnas.1734139100
In vivo regeneration of murine prostate from dissociated cell populations of postnatal epithelia and urogenital sinus mesenchyme
Abstract
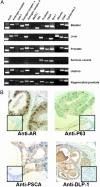
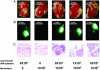
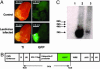
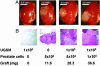
The existence of a postnatal prostate stem cell is supported by several types of evidence. Withdrawal of androgen leads to involution of the gland, but readdition can rapidly stimulate regeneration. Tissue fragments derived from mouse or rat prostatic epithelia from midgestation embryos or adult mice, when combined with tissue fragments from urogenital sinus mesenchyme and grafted under the kidney capsule, can regenerate prostatic structures. Indirect evidence supports that the stem cell population is contained within the basal layer. Purified prostatic stem cell preparations would be useful to define the physical and functional properties required for regeneration and to compare with cells that accumulate during abnormal growth states, like prostate cancer. We have developed a regeneration system using dissociated cell populations of postnatal prostate epithelia and embryonic urogenital sinus mesenchyme. Efficient in vivo regeneration of prostatic structures in the subcapsular space of the kidney was observed within 4-8 wk with as few as 103 epithelial cells from prostates derived from donors 10 d to 6 wk of age. The regenerated structures show a branching tubular epithelial morphology, with expression of a panel of markers consistent with prostate development. Donor epithelial populations can be readily infected with GFP expressing lentiviral vectors to provide integration markers and easy visualization. The cell preparations of urogenital sinus mesenchyme can be expanded in short-term in vitro culture while their inductive capabilities are retained. Further definition of the subpopulation of prostate epithelial cells containing the regeneration activity should be possible with such technologies.
Figures



References
-
- Weissman, I. L., Anderson, D. J. & Gage, F. (2001) Annu. Rev. Cell Dev. Biol. 17, 387-403. - PubMed
-
- Jackson, K. A., Majka, S. M., Wulf, G. G. & Goodell, M. A. (2002) J. Cell Biochem. 38, Suppl., 1-6. - PubMed
-
- Reya, T., Morrison, S. J., Clarke, M. F. & Weissman, I. L. (2001) Nature 414, 105-111. - PubMed
-
- Ramalho-Santos, M., Yoon, S., Matsuzaki, Y., Mulligan, R. C. & Melton, D. A. (2002) Science 298, 597-600. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

