Targeted gene evolution in Escherichia coli using a highly error-prone DNA polymerase I
- PMID: 12909725
- PMCID: PMC187833
- DOI: 10.1073/pnas.1333928100
Targeted gene evolution in Escherichia coli using a highly error-prone DNA polymerase I
Abstract
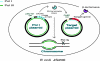
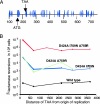
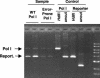
We present a system for random mutagenesis in Escherichia coli for the evolution of targeted genes. To increase error rates of DNA polymerase I (Pol I) replication, we introduced point mutations in three structural domains that govern Pol I fidelity. Expression of error-prone Pol I in vivo results in strong mutagenesis of a target sequence encoded in a Pol I-dependent plasmid (8.1 x 10-4 mutations per bp, an 80,000-fold increase), with a preference for plasmid relative to chromosome sequence. Mutagenesis is maximal in cultures maintained at stationary phase. Mutations are evenly distributed and show a variety of base pair substitutions, predominantly transitions. Mutagenesis extends at least 3 kb beyond the 400-500 nt reportedly synthesized by Pol I. We demonstrate that our error-prone Pol I can be used to generate enzymes with distinct properties by generating TEM-1 beta-lactamase mutants able to hydrolyze a third-generation lactam antibiotic, aztreonam. Three different mutations contribute to aztreonam resistance. Two are found in the extended-spectrum beta-lactamases most frequently identified in clinical isolates, and the third (G276R) has not been previously described. Our system of targeted mutagenesis in E. coli should have an impact on enzyme-based applications in areas such as synthetic chemistry, gene therapy, and molecular biology. Given the structural conservation between polymerases, this work should also provide a reference for altering the fidelity of other polymerases.
Figures

References
-
- Kunkel, T. A. & Bebenek, K. (2000) Annu. Rev. Biochem. 69, 497–529. - PubMed
-
- Kornberg, A. & Baker, T. A. (1992) DNA Replication (Freeman, New York).
-
- Patel, P. H., Suzuki, M., Adman, E., Shinkai, A. & Loeb, L. A. (2001) J. Mol. Biol. 308, 823–837. - PubMed
-
- Cadwell, R. C. & Joyce, G. F. (1994) PCR Methods Appl. 3, S136–40. - PubMed
-
- Camps, M. & Loeb, L. A. (2003) in Directed Enzyme Evolution Screening and Selection Methods, ed. Walker, J. M. (Humana, Totowa, NJ), pp. 11–18.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

