Serial cardiac magnetic resonance imaging of injected mesenchymal stem cells
- PMID: 12912822
- PMCID: PMC1490325
- DOI: 10.1161/01.CIR.0000084537.66419.7A
Serial cardiac magnetic resonance imaging of injected mesenchymal stem cells
Abstract
Background: Delivery and tracking of endomyocardial stem cells are limited by the inability to image transplanted cells noninvasively in the beating heart. We hypothesized that mesenchymal stem cells (MSCs) could be labeled with a iron fluorophore particle (IFP) to provide MRI contrast in vivo to assess immediate and long-term localization.
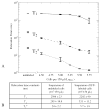
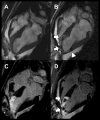
Methods and results: MSCs were isolated from swine. Short-term incubation of MSCs with IFP resulted in dose-dependent and efficient labeling. Labeled cells remained viable for multiple passages and retained in vitro proliferation and differentiation capacity. Labeled MSCs (10(4) to 10(6) cells/150 microL) were injected percutaneously into normal and freshly infarcted myocardium in swine. One, 3, and 1 animals underwent serial cardiac MRI (1.5T) for 4, 8, and 21 days, respectively. MRI contrast properties were measured both in vivo and in vitro for cells embedded in agar. Injection sites containing as few as 10(5) MSCs could be detected and contained intact IFP-bearing MSCs on histology.
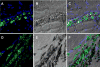
Conclusions: IFP labeling of MSCs imparts useful MRI contrast, enabling ready detection in the beating heart on a conventional cardiac MR scanner after transplantation into normal and infarcted myocardium. The dual-labeled MSCs can be identified at locations corresponding to injection sites, both ex vivo using fluorescence microscopy and in vivo using susceptibility contrast on MRI. This technology may permit effective in vivo study of stem cell retention, engraftment, and migration.
Figures



References
-
- Reinlib L, Field L. Cell transplantation as future therapy for cardiovascular disease? A workshop of the National Heart, Lung, and Blood Institute. Circulation. 2000;101:E182–E127. - PubMed
-
- Huhn RD, Tisdale JF, Agricola B, et al. Retroviral marking and transplantation of rhesus hematopoietic cells by nonmyeloablative conditioning. Hum Gene Ther. 1999;10:1783–1790. - PubMed
-
- Shi PA, Hematti P, von Kalle C, et al. Genetic marking as an approach to studying in vivo hematopoiesis: progress in the non-human primate model. Oncogene. 2002;21:3274–3283. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

