Monocyte chemoattractant protein 1 (MCP-1) released from Helicobacter pylori stimulated gastric epithelial cells induces cyclooxygenase 2 expression and activation in T cells
- PMID: 12912855
- PMCID: PMC1773780
- DOI: 10.1136/gut.52.9.1257
Monocyte chemoattractant protein 1 (MCP-1) released from Helicobacter pylori stimulated gastric epithelial cells induces cyclooxygenase 2 expression and activation in T cells
Abstract
Background: and aims: To clarify the interaction between gastric epithelial and mucosal T cells, we examined the role of cytokines released from epithelial cells in response to Helicobacter pylori water extract protein (HPWEP) in regulating T cell cyclooxygenase 2 (COX-2) expression and activation.
Methods: Media from MKN-28 cells incubated with HPWEP for 48 hours were added to Jurkat T cells and human peripheral T cells. C-C and CXC chemokine concentrations in MKN-28 cell media, and COX-2 expression, interferon gamma (IFN-gamma), and interleukin (IL)-4 secretions in T cells were determined by western blot analysis and ELISA methods. Distributions of COX-2 positive T cells and monocyte chemoattractant protein 1 (MCP-1) in tissue specimens with H pylori associated gastritis were determined as single or double labelling by immunohistochemistry.
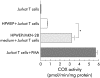
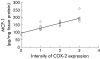
Results: MCP-1, IL-7, IL-8, and RANTES were detected in media from MKN-28 cells incubated with HPWEP. Media as a whole, and MCP-1 alone, stimulated COX-2 expression and peripheral T cell proliferation. Anti-MCP-1 antibody inhibited media stimulated COX-2 mRNA expression in Jurkat T cells. Media stimulated IFN-gamma but not IL-4 secretion from peripheral T cells, while MCP-1 stimulated IL-4 but not IFN-gamma secretion. Both stimulated cytokine release, and peripheral T cell proliferation was partially inhibited by NS-398, a specific COX-2 inhibitor. In mucosa with gastritis, COX-2 was expressed in T cells and MCP-1 was localised mainly in epithelial and mononuclear cells. MCP-1 levels and the intensity of COX-2 expression in tissue samples were closely related.
Conclusions: Cytokines such as MCP-1, released from gastric epithelial cells in response to HPWEP, seem to modulate T cell immune responses, at least in part via COX-2 expression.
Figures






Similar articles
-
Helicobacter pylori-induced expression of interleukin-8 and cyclooxygenase-2 in AGS gastric epithelial cells: mediation by nuclear factor-kappaB.Scand J Gastroenterol. 2001 Jul;36(7):706-16. doi: 10.1080/003655201300191969. Scand J Gastroenterol. 2001. PMID: 11444469
-
gammadelta T cells increase with gastric mucosal interleukin (IL)-7, IL-1beta, and Helicobacter pylori urease specific immunoglobulin levels via CCR2 upregulation in Helicobacter pylori gastritis.J Gastroenterol Hepatol. 2006 Jan;21(1 Pt 1):32-40. doi: 10.1111/j.1440-1746.2005.04148.x. J Gastroenterol Hepatol. 2006. PMID: 16706809
-
Localisation of cyclooxygenase 1 and cyclooxygenase 2 in Helicobacter pylori related gastritis and gastric ulcer tissues in humans.Gut. 2000 Jun;46(6):782-9. doi: 10.1136/gut.46.6.782. Gut. 2000. PMID: 10807888 Free PMC article.
-
Helicobacter pylori and gastric inflammation.Br Med Bull. 1998;54(1):139-50. doi: 10.1093/oxfordjournals.bmb.a011664. Br Med Bull. 1998. PMID: 9604438 Review.
-
[Inflammatory and immune responses of gastric mucosa on Helicobacter pylori infection].Klin Med (Mosk). 2000;78(11):9-13. Klin Med (Mosk). 2000. PMID: 11232538 Review. Russian. No abstract available.
Cited by
-
Monocyte chemoattractant protein 1 and CD40 ligation have a synergistic effect on vascular endothelial growth factor production through cyclooxygenase 2 upregulation in gastric cancer.J Gastroenterol. 2008;43(3):216-24. doi: 10.1007/s00535-007-2151-8. Epub 2008 Mar 29. J Gastroenterol. 2008. PMID: 18373164
-
Campylobacter jejuni induces secretion of proinflammatory chemokines from human intestinal epithelial cells.Infect Immun. 2005 Jul;73(7):4437-40. doi: 10.1128/IAI.73.7.4437-4440.2005. Infect Immun. 2005. PMID: 15972545 Free PMC article.
-
Serum cytokines MCP-1 and GCS-F as potential biomarkers in pediatric inflammatory bowel disease.PLoS One. 2023 Nov 3;18(11):e0288147. doi: 10.1371/journal.pone.0288147. eCollection 2023. PLoS One. 2023. PMID: 37922289 Free PMC article.
-
MCP-1: Function, regulation, and involvement in disease.Int Immunopharmacol. 2021 Dec;101(Pt B):107598. doi: 10.1016/j.intimp.2021.107598. Epub 2021 May 20. Int Immunopharmacol. 2021. PMID: 34233864 Free PMC article. Review.
-
Evodiamine Inhibits Helicobacter pylori Growth and Helicobacter pylori-Induced Inflammation.Int J Mol Sci. 2021 Mar 25;22(7):3385. doi: 10.3390/ijms22073385. Int J Mol Sci. 2021. PMID: 33806161 Free PMC article.
References
-
- Keates S, Sougioultzis S, Keates AC, et al. Cag+ Helicobacter pylori induce transactivation of the epidermal growth factor receptor in AGS gastric epithelial cells. J Biol Chem 2001;276:48127–34. - PubMed
-
- Crowe SE, Alvarez L, Dytoc M, et al. Expression of interleukin 8 and CD 54 by human gastric epithelium after Helicobacter pylori infection in vitro. Gastroenterology 1995;108:65–74. - PubMed
-
- D’Elios MM, Manghetti M, Carli MD, et al. T helper 1 effector cells specific for Helicobacter pylori in the gastric antrum of patients with peptic ulcer disease. J Immunol 1997;158:962–7. - PubMed
-
- Abe N, Katamura K, Shintaku N, et al. Prostaglandin E2 and IL-4 provide native CD4+ T cells with distinct inhibitory signals for the priming of IFN-γ production. Cell Immunol 1997;181:86–92. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous
