Vascular endothelial growth factor and receptor interaction is a prerequisite for murine hepatic fibrogenesis
- PMID: 12912869
- PMCID: PMC1773781
- DOI: 10.1136/gut.52.9.1347
Vascular endothelial growth factor and receptor interaction is a prerequisite for murine hepatic fibrogenesis
Abstract
Background: It has been shown that expression of the potent angiogenic factor, vascular endothelial growth factor (VEGF), and its receptors, flt-1 (VEGFR-1) and KDR/Flk-1 (VEGFR-2), increased during the development of liver fibrosis.
Aims: To elucidate the in vivo role of interaction between VEGF and its receptors in liver fibrogenesis.
Methods: A model of CCl(4) induced hepatic fibrosis was used to assess the role of VEGFR-1 and VEGFR-2 by means of specific neutralising monoclonal antibodies (R-1mAb and R-2mAb, respectively). R-1mAb and R-2mAb were administered after two weeks of treatment with CCl(4), and indices of fibrosis were assessed at eight weeks.
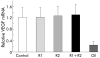
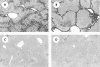
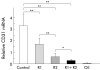
Results: Hepatic VEGF mRNA expression significantly increased during the development of liver fibrosis. Both R-1mAb and R-2mAb treatments significantly attenuated the development of fibrosis associated with suppression of neovascularisation in the liver. Hepatic hydroxyproline and serum fibrosis markers were also suppressed. Furthermore, the number of alpha-smooth muscle actin positive cells and alpha1(I)-procollagen mRNA expression were significantly suppressed by R-1mAb and R-2mAb treatment. The inhibitory effect of R-2mAb was more potent than that of R-1mAb, and combination treatment with both mAbs almost completely attenuated fibrosis development. Our in vitro study showed that VEGF treatment significantly stimulated proliferation of both activated hepatic stellate cells (HSC) and sinusoidal endothelial cells (SEC). VEGF also significantly increased alpha1(I)-procollagen mRNA expression in activated HSC.
Conclusions: These results suggest that the interaction of VEGF and its receptor, which reflected the combined effects of both on HSC and SEC, was a prerequisite for liver fibrosis development.
Figures
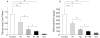



References
-
- Friedman SL. Cytokines and fibrogenesis. Semin Liver Dis 1999;19:129–40. - PubMed
-
- Park YN, Yang CP, Fernandez GJ, et al. Neoangiogenesis and sinusoidal “capillarization” in dysplastic nodules of the liver. Am J Surg Pathol 1998;22:656–62. - PubMed
-
- Schaffner F, Popper H. Capillarization of hepatic sinusoids in man. Gastroenterology 1963;44:239–42. - PubMed
-
- Tanigawa N, Lu C, Mitsui T, et al. Quantitation of sinusoid-like vessels in hepatocellular carcinoma: its clinical and prognostic significance. Hepatology 1997;26:1216–23. - PubMed
-
- Wisse E, De Zanger RB, Charels K, et al. The liver sieve: considerations concerning the structure and function of endothelial fenestrae, the sinusoidal wall and the space of Disse. Hepatology 1985;5:683–92. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
