Activation of phospholipase D by the small GTPase Sar1p is required to support COPII assembly and ER export
- PMID: 12912905
- PMCID: PMC175780
- DOI: 10.1093/emboj/cdg390
Activation of phospholipase D by the small GTPase Sar1p is required to support COPII assembly and ER export
Abstract
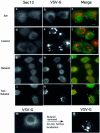
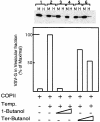
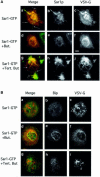
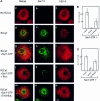
The small GTPase Sar1p controls the assembly of the cytosolic COPII coat that mediates export from the endoplasmic reticulum (ER). Here we demonstrate that phospholipase D (PLD) activation is required to support COPII-mediated ER export. PLD activity by itself does not lead to the recruitment of COPII to the membranes or ER export. However, PLD activity is required to support Sar1p-dependent membrane tubulation, the subsequent Sar1p-dependent recruitment of Sec23/24 and Sec13/31 COPII complexes to ER export sites and ER export. Sar1p recruitment to the membrane is PLD independent, yet activation of Sar1p is required to stimulate PLD activity on ER membranes, thus PLD is temporally regulated to support ER export. Regulated modification of membrane lipid composition is required to support the cooperative interactions that enable selective transport, as we demonstrate here for the mammalian COPII coat.
Figures


References
-
- Allan D. (1996) Mapping the lipid distribution in the membranes of BHK cells (mini-review). Mol. Membr. Biol., 13, 81–84. - PubMed
-
- Antonny B. and Schekman,R. (2001) ER export: public transportation by the COPII coach. Curr. Opin. Cell Biol., 13, 438–443. - PubMed
-
- Aridor M. and Balch,W.E. (2000) Kinase signaling initiates coat complex II (COPII) recruitment and export from the mammalian endoplasmic reticulum. J. Biol. Chem., 275, 35673–35676. - PubMed
-
- Aridor M. and Traub,L.M. (2002) Cargo selection in vesicular transport: the making and breaking of a coat. Traffic, 3, 537–546. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

