GCNF-dependent repression of BMP-15 and GDF-9 mediates gamete regulation of female fertility
- PMID: 12912906
- PMCID: PMC175795
- DOI: 10.1093/emboj/cdg405
GCNF-dependent repression of BMP-15 and GDF-9 mediates gamete regulation of female fertility
Abstract
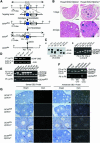
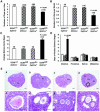
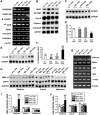
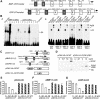
To determine the function of germ cell nuclear factor (GCNF) in female reproduction, we generated an oocyte-specific GCNF knockout mouse model (GCNF(fl/fl)Zp3Cre(+)). These mice displayed hypofertility due to prolonged diestrus phase of the estrous cycle and aberrant steroidogenesis. These reproductive defects were secondary to a primary defect in the oocytes, in which expression of the paracrine transforming growth factor-beta signaling molecules, bone morphogenetic protein 15 (BMP-15) and growth differentiation factor 9 (GDF-9), were up-regulated in GCNF(fl/fl)Zp3Cre(+) females at diestrus. This was a direct effect of GCNF, as molecular studies showed that GCNF bound to DR0 elements within the BMP-15 and GDF-9 gene promoters and repressed their reporter activities. Consistent with these findings, abnormal double-oocyte follicles, indicative of aberrant BMP-15/GDF-9 expression, were observed in GCNF(fl/fl)Zp3Cre(+) females. The Cre/loxP knockout of GCNF in the oocyte has uncovered a new regulatory pathway in ovarian function. Our results show that GCNF directly regulates paracrine communication between the oocyte and somatic cells by regulating the expression of BMP-15 and GDF-9, to affect female fertility.
Figures
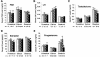
References
-
- Agoulnik I.Y., Cho,Y., Niederberger,C., Kieback,D.G. and Cooney,A.J. (1998) Cloning, expression analysis and chromosomal localization of the human nuclear receptor gene GCNF. FEBS Lett., 424, 73–78. - PubMed
-
- Braat A.K., Zandbergen,M.A., De Vries,E., Van Der Burg,B., Bogerd,J. and Goos,H.J. (1999) Cloning and expression of the zebrafish germ cell nuclear factor. Mol. Reprod. Dev., 53, 369–375. - PubMed
-
- Chen F., Cooney,A.J., Wang,Y., Law,S.W. and O’Malley,B.W. (1994) Cloning of a novel orphan receptor (GCNF) expressed during germ cell development. Mol. Endocrinol., 8, 1434–1444. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

