A signalling role for 4-hydroxy-2-nonenal in regulation of mitochondrial uncoupling
- PMID: 12912909
- PMCID: PMC175801
- DOI: 10.1093/emboj/cdg412
A signalling role for 4-hydroxy-2-nonenal in regulation of mitochondrial uncoupling
Abstract
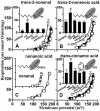
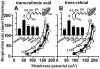
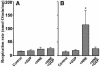
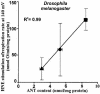
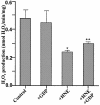
Oxidative stress and mitochondrial dysfunction are associated with disease and aging. Oxidative stress results from overproduction of reactive oxygen species (ROS), often leading to peroxidation of membrane phospholipids and production of reactive aldehydes, particularly 4-hydroxy-2-nonenal. Mild uncoupling of oxidative phosphorylation protects by decreasing mitochondrial ROS production. We find that hydroxynonenal and structurally related compounds (such as trans-retinoic acid, trans-retinal and other 2-alkenals) specifically induce uncoupling of mitochondria through the uncoupling proteins UCP1, UCP2 and UCP3 and the adenine nucleotide translocase (ANT). Hydroxynonenal-induced uncoupling was inhibited by potent inhibitors of ANT (carboxyatractylate and bongkrekate) and UCP (GDP). The GDP-sensitive proton conductance induced by hydroxynonenal correlated with tissue expression of UCPs, appeared in yeast mitochondria expressing UCP1 and was absent in skeletal muscle mitochondria from UCP3 knockout mice. The carboxyatractylate-sensitive hydroxynonenal stimulation correlated with ANT content in mitochondria from Drosophila melanogaster expressing different amounts of ANT. Our findings indicate that hydroxynonenal is not merely toxic, but may be a biological signal to induce uncoupling through UCPs and ANT and thus decrease mitochondrial ROS production.
Figures


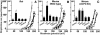


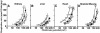


References
-
- Alvarez R., de Andres,J., Yubero,P., Vinas,O., Mampel,T., Iglesias,R., Giralt,M. and Villarroya,F. (1995) A novel regulatory pathway of brown fat thermogenesis. Retinoic acid is a transcriptional activator of the mitochondrial uncoupling protein gene. J. Biol. Chem., 270, 5666–5673. - PubMed
-
- Boss O., Samec,S., Paoloni-Giacobino,A., Rossier,C., Dulloo,A., Seydoux,J., Muzzin,P. and Giacobino,J.-P. (1997) Uncoupling protein-3: a new member of the mitochondrial carrier family with tissue-specific expression. FEBS Lett., 408, 39–42. - PubMed
-
- Bouillaud F., Couplan,E., Pecqueur,C. and Ricquier,D. (2001) Homologues of the uncoupling protein from brown adipose tissue (UCP1): UCP2, UCP3, BMCP1 and UCP4. Biochim. Biophys. Acta, 1504, 107–119. - PubMed
-
- Brand M.D. (1995) Measurement of mitochondrial protonmotive force. In Brown,G.C. and Cooper,C.E. (eds), Bioenergetics—A Practical Approach. IRL Press, Oxford, UK, pp. 39–62.
-
- Brand M.D., Pamplona,R., Portero-Otin,M., Requena,J.R., Roebuck,S.J., Buckingham,J.A., Clapham,J.C. and Cadenas,S. (2002) Oxidative damage and phospholipid fatty acyl composition in skeletal muscle mitochondria from mice underexpressing or overexpressing uncoupling protein 3. Biochem. J., 368, 597–603. - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

