Pak1 and PIX regulate contact inhibition during epithelial wound healing
- PMID: 12912914
- PMCID: PMC175788
- DOI: 10.1093/emboj/cdg398
Pak1 and PIX regulate contact inhibition during epithelial wound healing
Abstract
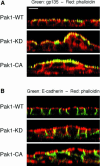
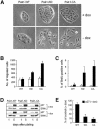
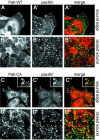
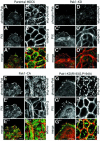
Wound healing in epithelia requires coordinated cell migration and proliferation regulated by signaling mechanisms that are poorly understood. Here we show that epithelial cells expressing constitutively active or kinase-dead mutants of the Rac/Cdc42 effector Pak1 fail to undergo growth arrest upon wound closure. Strikingly, this phenotype is only observed when the Pak1 kinase mutants are expressed in cells possessing a free lateral surface, i.e. one that is not engaged in contact with neighboring cells. The Pak1 kinase mutants perturb contact inhibition by a mechanism that depends on the Pak-interacting Rac-GEF PIX. In control cells, endogenous activated Pak and PIX translocate from focal complexes to cell-cell contacts during wound closure. This process is abrogated in cells expressing Pak1 kinase mutants. In contrast, Pak1 mutants rendered defective in PIX binding do not impede translocation of activated Pak and PIX, and exhibit normal wound healing. Thus, recruitment of activated Pak and PIX to cell-cell contacts is pivotal to transduction of growth-inhibitory signals from neighboring cells in epithelial wound healing.
Figures




References
-
- Bagrodia S. and Cerione,R.A. (1999) Pak to the future. Trends Cell Biol., 9, 350–355. - PubMed
-
- Bagrodia S., Taylor,S.J., Jordon,K.A., Van Aelst,L. and Cerione,R.A. (1998) A novel regulator of p21-activated kinases. J. Biol. Chem., 273, 23633–23636. - PubMed
-
- Bagrodia S., Bailey,D., Lenard,Z., Hart,M., Guan,J.L., Premont,R.T., Taylor,S.J. and Cerione,R.A. (1999) A tyrosine-phosphorylated protein that binds to an important regulatory region on the cool family of p21-activated kinase-binding proteins. J. Biol. Chem., 274, 22393–22400. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

