The in vivo roles of STEF/Tiam1, Rac1 and JNK in cortical neuronal migration
- PMID: 12912917
- PMCID: PMC175802
- DOI: 10.1093/emboj/cdg413
The in vivo roles of STEF/Tiam1, Rac1 and JNK in cortical neuronal migration
Abstract
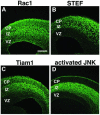
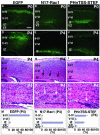
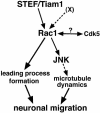
The coordinated migration of neurons is a pivotal step for functional architectural formation of the mammalian brain. To elucidate its molecular mechanism, gene transfer by means of in utero electroporation was applied in the developing murine brain, revealing the crucial roles of Rac1, its activators, STEF/Tiam1, and its downstream molecule, c-Jun N-terminal kinase (JNK), in the cerebral cortex. Functional repression of these molecules resulted in inhibition of radial migration of neurons without affecting their proper differentiation. Interestingly, distinct morphological phenotypes were observed; suppression of Rac1 activity caused loss of the leading process, whereas repression of JNK activity did not, suggesting the complexity of the signaling cascade. In cultured neurons from the intermediate zone, activated JNK was detected along microtubules in the processes. Application of a JNK inhibitor caused irregular morphology and increased stable microtubules in processes, and decreased phosphorylation of microtubule associated protein 1B, raising a possibility of the involvement of JNK in controlling tubulin dynamics in migrating neurons. Our data thus provide important clues for understanding the intracellullar signaling machinery for cortical neuronal migration.
Figures





References
-
- Bulinski J.C. and Gundersen,G.G. (1991) Stabilization of post-translational modification of microtubules during cellular morphogenesis. BioEssays, 13, 285–293. - PubMed
-
- Cheng A., Krueger,B.K. and Bambrick,L.L. (1999) MAP5 expression in proliferating neuroblasts. Brain Res. Dev. Brain Res., 113, 107–113. - PubMed
-
- Derijard B., Hibi,M., Wu,I.H., Barrett,T., Su,B., Deng,T., Karin,M. and Davis,R.J. (1994) JNK1: a protein kinase stimulated by UV light and Ha-Ras that binds and phosphorylates the c-Jun activation domain. Cell, 76, 1025–1037. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

