GATA-6 maintains BMP-4 and Nkx2 expression during cardiomyocyte precursor maturation
- PMID: 12912923
- PMCID: PMC175790
- DOI: 10.1093/emboj/cdg400
GATA-6 maintains BMP-4 and Nkx2 expression during cardiomyocyte precursor maturation
Abstract
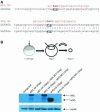
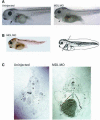
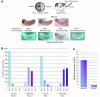
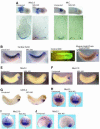
GATA-6 is expressed in presumptive cardiac mesoderm before gastrulation, but its role in heart development has been unclear. Here we show that Xenopus and zebrafish embryos, injected with antisense morpholino oligonucleotides designed specifically to knock-down translation of GATA-6 protein, are severely compromised for heart development. Injected embryos express greatly reduced levels of contractile machinery genes and, at the same stage, of regulatory genes such as bone morphogenetic protein-4 (BMP-4) and the Nkx2 family. In contrast, initial BMP and Nkx2 expression is normal, suggesting a maintenance role for GATA-6. Endoderm is critical for heart formation in several vertebrates including Xenopus, and separate perturbation of GATA-6 expression in the deep anterior endoderm and in the overlying heart mesoderm shows that GATA-6 is required in both for cardiogenesis. The GATA-6 requirement in cardiac mesoderm was confirmed in zebrafish, an organism in which endoderm is thought not to be necessary for heart formation. We therefore conclude that proper maturation of cardiac mesoderm requires GATA-6, which functions to maintain BMP-4 and Nkx2 expression.
Figures

Similar articles
-
A role for GATA-4/5/6 in the regulation of Nkx2.5 expression with implications for patterning of the precardiac field.Dev Biol. 1999 Dec 1;216(1):57-71. doi: 10.1006/dbio.1999.9469. Dev Biol. 1999. PMID: 10588863
-
Suppression of GATA factor activity causes axis duplication in Xenopus.Development. 1998 Dec;125(23):4595-605. doi: 10.1242/dev.125.23.4595. Development. 1998. PMID: 9806909
-
A role for the homeobox gene Xvex-1 as part of the BMP-4 ventral signaling pathway.Mech Dev. 1999 Aug;86(1-2):99-111. doi: 10.1016/s0925-4773(99)00120-3. Mech Dev. 1999. PMID: 10446269
-
GATA transcription factors and cardiac development.Semin Cell Dev Biol. 1999 Feb;10(1):85-91. doi: 10.1006/scdb.1998.0281. Semin Cell Dev Biol. 1999. PMID: 10355032 Review.
-
Early endoderm development in vertebrates: lineage differentiation and morphogenetic function.Curr Opin Genet Dev. 2003 Aug;13(4):393-400. doi: 10.1016/s0959-437x(03)00085-6. Curr Opin Genet Dev. 2003. PMID: 12888013 Review.
Cited by
-
Heart function and thoracic aorta gene expression profiling studies of ginseng combined with different herbal medicines in eNOS knockout mice.Sci Rep. 2017 Nov 13;7(1):15431. doi: 10.1038/s41598-017-15819-2. Sci Rep. 2017. PMID: 29133875 Free PMC article.
-
ARF alters PAF1 complex integrity to selectively repress oncogenic transcription programs upon p53 loss.Mol Cell. 2024 Dec 5;84(23):4538-4557.e12. doi: 10.1016/j.molcel.2024.10.020. Epub 2024 Nov 11. Mol Cell. 2024. PMID: 39532099 Free PMC article.
-
Dynamic patterning at the pylorus: formation of an epithelial intestine-stomach boundary in late fetal life.Dev Dyn. 2009 Dec;238(12):3205-17. doi: 10.1002/dvdy.22134. Dev Dyn. 2009. PMID: 19877272 Free PMC article.
-
Mechanisms of cardiogenesis in cardiovascular progenitor cells.Int Rev Cell Mol Biol. 2012;293:195-267. doi: 10.1016/B978-0-12-394304-0.00012-9. Int Rev Cell Mol Biol. 2012. PMID: 22251563 Free PMC article. Review.
-
Identification of a Tbx1/Tbx2/Tbx3 genetic pathway governing pharyngeal and arterial pole morphogenesis.Hum Mol Genet. 2012 Mar 15;21(6):1217-29. doi: 10.1093/hmg/ddr553. Epub 2011 Nov 24. Hum Mol Genet. 2012. PMID: 22116936 Free PMC article.
References
-
- Alexander J. and Stainier,D.Y.R. (1999) A molecular pathway leading to endoderm formation in zebrafish. Curr. Biol., 9, 1147–1157. - PubMed
-
- Andree B., Duprez,D., Vorbusch,B., Arnold,H.H. and Brand,T. (1998) BMP-2 induces ectopic expression of cardiac lineage markers and interferes with somite formation in chicken embryos. Mech. Dev., 70, 119–131. - PubMed
-
- Attisano L. and Wrana,J.L. (2000) Smads as transcriptional co-modulators. Curr. Opin. Cell Biol., 12, 235–243. - PubMed
-
- Blokzijl A., ten Dijke,P. and Ibanez,C.F. (2002) Physical and functional interaction between GATA-3 and Smad3 allows TGF-β regulation of GATA target genes. Curr. Biol., 12, 35–45. - PubMed
-
- Brewer A. et al. (1999) The human and mouse GATA-6 genes utilize two promoters and two initiation codons. J. Biol. Chem., 274, 38004–38016. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

