A role of the fast ATP-gated P2X1 cation channel in thrombosis of small arteries in vivo
- PMID: 12913094
- PMCID: PMC2194166
- DOI: 10.1084/jem.20030144
A role of the fast ATP-gated P2X1 cation channel in thrombosis of small arteries in vivo
Abstract
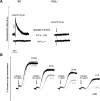
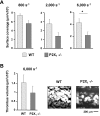
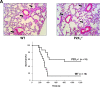
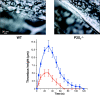
The P2X1 receptor is a fast ATP-gated cation channel expressed in blood platelets, where its role has been difficult to assess due to its rapid desensitization and the lack of pharmacological tools. In this paper, we have used P2X1-/- and wild-type mouse platelets, treated with apyrase to prevent desensitization, to demonstrate the function of P2X1 in the response to thrombogenic stimuli. In vitro, the collagen-induced aggregation and secretion of P2X1-deficient platelets was decreased, as was adhesion and thrombus growth on a collagen-coated surface, particularly when the wall shear rate was elevated. In vivo, the functional role of P2X1 could be demonstrated using two models of platelet-dependent thrombotic occlusion of small arteries, in which blood flow is characterized by a high shear rate. The mortality of P2X1-/- mice in a model of systemic thromboembolism was reduced and the size of mural thrombi formed after a laser-induced vessel wall injury was decreased as compared with normal mice, whereas the time for complete thrombus removal was shortened. Overall, the P2X1 receptor appears to contribute to the formation of platelet thrombi, particularly in arteries in which shear forces are high.
Figures


References
-
- Gachet, C. 2001. ADP receptors of platelets and their inhibition. Thromb. Haemost. 86:222–232. - PubMed
-
- Fabre, J.E., M. Nguyen, A. Latour, J.A. Keifer, L.P. Audoly, T.M. Coffman, and B.H. Koller. 1999. Decreased platelet aggregation, increased bleeding time and resistance to thromboembolism in P2Y1-deficient mice. Nat. Med. 5:1199–1202. - PubMed
-
- Hollopeter, G., H. Jantzen, D. Vincent, G. Li, L. England, V. Ramakrishan, R. Yang, P. Nurden, A. Nurden, D. Julius, and P. Conley. 2001. Identification of the platelet ADP receptor targeted by antithrombotic drugs. Nature. 409:202–207. - PubMed
-
- Foster, C.J., D.M. Prosser, J.M. Agans, Y. Zhai, M.D. Smith, J.E. Lachowicz, F.L. Zhang, E. Gustafson, F.J. Monsma, Jr., M.T. Wiekowski, et al. 2001. Molecular identification and characterization of the platelet ADP receptor targeted by thienopyridine antithrombotic drugs. J. Clin. Invest. 107:1591–1598. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

