Stem cells of the skin epithelium
- PMID: 12913119
- PMCID: PMC304094
- DOI: 10.1073/pnas.1734203100
Stem cells of the skin epithelium
Abstract
Tissue stem cells form the cellular base for organ homeostasis and repair. Stem cells have the unusual ability to renew themselves over the lifetime of the organ while producing daughter cells that differentiate into one or multiple lineages. Difficult to identify and characterize in any tissue, these cells are nonetheless hotly pursued because they hold the potential promise of therapeutic reprogramming to grow human tissue in vitro, for the treatment of human disease. The mammalian skin epithelium exhibits remarkable turnover, punctuated by periods of even more rapid production after injury due to burn or wounding. The stem cells responsible for supplying this tissue with cellular substrate are not yet easily distinguishable from neighboring cells. However, in recent years a significant body of work has begun to characterize the skin epithelial stem cells, both in tissue culture and in mouse and human skin. Some epithelial cells cultured from skin exhibit prodigious proliferative potential; in fact, for >20 years now, cultured human skin has been used as a source of new skin to engraft onto damaged areas of burn patients, representing one of the first therapeutic uses of stem cells. Cell fate choices, including both self-renewal and differentiation, are crucial biological features of stem cells that are still poorly understood. Skin epithelial stem cells represent a ripe target for research into the fundamental mechanisms underlying these important processes.
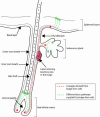
Figures

References
-
- Fuchs, E. & Cleveland, D. W. (1998) Science 279, 514-519. - PubMed
-
- Kalinin, A. E., Kajava, A. V. & Steinert, P. M. (2002) BioEssays 24, 789-800. - PubMed
-
- Gandarillas, A. (2000) Exp. Gerontol. 35, 53-62. - PubMed
-
- Potten, C. S. (1975) Br. J. Dermatol. 93, 649-658. - PubMed
-
- Rheinwald, J. G. & Green, H. (1975) Cell 6, 331-343. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

