TaVRT-1, a putative transcription factor associated with vegetative to reproductive transition in cereals
- PMID: 12913142
- PMCID: PMC181271
- DOI: 10.1104/pp.103.023523
TaVRT-1, a putative transcription factor associated with vegetative to reproductive transition in cereals
Abstract
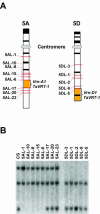
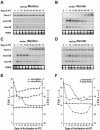
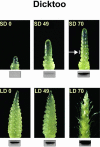
The molecular genetics of vernalization, defined as the promotion of flowering by cold treatment, is still poorly understood in cereals. To better understand this mechanism, we cloned and characterized a gene that we named TaVRT-1 (wheat [Triticum aestivum] vegetative to reproductive transition-1). Molecular and sequence analyses indicated that this gene encodes a protein homologous to the MADS-box family of transcription factors that comprises certain flowering control proteins in Arabidopsis. Mapping studies have localized this gene to the Vrn-1 regions on the long arms of homeologous group 5 chromosomes, regions that are associated with vernalization and freezing tolerance (FT) in wheat. The level of expression of TaVRT-1 is positively associated with the vernalization response and transition from vegetative to reproductive phase and is negatively associated with the accumulation of COR genes and degree of FT. Comparisons among different wheat genotypes, near-isogenic lines, and cereal species, which differ in their vernalization response and FT, indicated that the gene is inducible only in those species that require vernalization, whereas it is constitutively expressed in spring habit genotypes. In addition, experiments using both the photoperiod-sensitive barley (Hordeum vulgare cv Dicktoo) and short or long day de-acclimated wheat revealed that the expression of TaVRT-1 is also regulated by photoperiod. These expression studies indicate that photoperiod and vernalization may regulate this gene through separate pathways. We suggest that TaVRT-1 is a key developmental gene in the regulatory pathway that controls the transition from the vegetative to reproductive phase in cereals.
Figures





References
-
- Blázquez MA (2000) Flower development pathways. J Cell Sci 113: 3547–3548 - PubMed
-
- Blom N, Gammeltoft S, Brunak S (1999) Sequence and structure-based prediction of eukaryotic protein phosphorylation sites. J Mol Biol 294: 1351–1362 - PubMed
-
- Brule-Babel AL, Fowler DB (1988) Genetic control of cold hardiness and vernalization requirement in winter wheat. Crop Sci 28: 879–884
-
- Danyluk J, Sarhan F (1990) Differential mRNA transcription during the induction of freezing tolerance in spring and winter wheat. Plant Cell Physiol 31: 609–619
-
- Dubcovsky J, Lijavetzky D, Appendino L, Tranquilli G (1998) Comparative RFLP mapping of Triticum monococcum genes controlling vernalization requirement. Theor Appl Genet 97: 968–975
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

