Thermotolerant guard cell protoplasts of tree tobacco do not require exogenous hormones to survive in culture and are blocked from reentering the cell cycle at the G1-to-S transition
- PMID: 12913149
- PMCID: PMC181278
- DOI: 10.1104/pp.103.024067
Thermotolerant guard cell protoplasts of tree tobacco do not require exogenous hormones to survive in culture and are blocked from reentering the cell cycle at the G1-to-S transition
Abstract
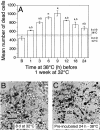
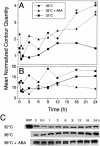
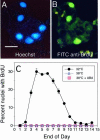
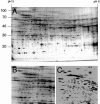
When guard cell protoplasts (GCPs) of tree tobacco [Nicotiana glauca (Graham)] are cultured at 32 degrees C with an auxin (1-napthaleneacetic acid) and a cytokinin (6-benzylaminopurine), they reenter the cell cycle, dedifferentiate, and divide. GCPs cultured similarly but at 38 degrees C and with 0.1 micro M +/- -cis,trans-abscisic acid (ABA) remain differentiated. GCPs cultured at 38 degrees C without ABA dedifferentiate partially but do not divide. Cell survival after 1 week is 70% to 80% under all of these conditions. In this study, we show that GCPs cultured for 12 to 24 h at 38 degrees C accumulate heat shock protein 70 and develop a thermotolerance that, upon transfer of cells to 32 degrees C, enhances cell survival but inhibits cell cycle reentry, dedifferentiation, and division. GCPs dedifferentiating at 32 degrees C require both 1-napthaleneacetic acid and 6-benzylaminopurine to survive, but thermotolerant GCPs cultured at 38 degrees C +/- ABA do not require either hormone for survival. Pulse-labeling experiments using 5-bromo-2-deoxyuridine indicate that culture at 38 degrees C +/- ABA prevents dedifferentiation of GCPs by blocking cell cycle reentry at G1/S. Cell cycle reentry at 32 degrees C is accompanied by loss of a 41-kD polypeptide that cross-reacts with antibodies to rat (Rattus norvegicus) extracellular signal-regulated kinase 1; thermotolerant GCPs retain this polypeptide. A number of polypeptides unique to thermotolerant cells have been uncovered by Boolean analysis of two-dimensional gels and are targets for further analysis. GCPs of tree tobacco can be isolated in sufficient numbers and with the purity required to study plant cell thermotolerance and its relationship to plant cell survival, growth, dedifferentiation, and division in vitro.
Figures


Similar articles
-
Heat reduces nitric oxide production required for auxin-mediated gene expression and fate determination in tree tobacco guard cell protoplasts.Plant Physiol. 2012 Aug;159(4):1608-23. doi: 10.1104/pp.112.200089. Epub 2012 Jun 22. Plant Physiol. 2012. PMID: 22730424 Free PMC article.
-
Heat suppresses activation of an auxin-responsive promoter in cultured guard cell protoplasts of tree tobacco.Plant Physiol. 2007 Oct;145(2):367-77. doi: 10.1104/pp.107.104646. Epub 2007 Aug 17. Plant Physiol. 2007. PMID: 17704234 Free PMC article.
-
Visualization of abscisic acid-perception sites on the plasma membrane of stomatal guard cells.Plant J. 2003 Jul;35(1):129-39. doi: 10.1046/j.1365-313x.2003.01782.x. Plant J. 2003. PMID: 12834408
-
Why are quiescent mesophyll protoplasts from Nicotiana sylvestris able to re-enter into the cell cycle and re-initiate a mitotic activity?Biochimie. 1993;75(7):539-45. doi: 10.1016/0300-9084(93)90059-2. Biochimie. 1993. PMID: 8268254 Review.
-
Protein phosphorylation in stomatal movement.Plant Signal Behav. 2014;9(11):e972845. doi: 10.4161/15592316.2014.972845. Plant Signal Behav. 2014. PMID: 25482764 Free PMC article. Review.
Cited by
-
Heat reduces nitric oxide production required for auxin-mediated gene expression and fate determination in tree tobacco guard cell protoplasts.Plant Physiol. 2012 Aug;159(4):1608-23. doi: 10.1104/pp.112.200089. Epub 2012 Jun 22. Plant Physiol. 2012. PMID: 22730424 Free PMC article.
-
Heat suppresses activation of an auxin-responsive promoter in cultured guard cell protoplasts of tree tobacco.Plant Physiol. 2007 Oct;145(2):367-77. doi: 10.1104/pp.107.104646. Epub 2007 Aug 17. Plant Physiol. 2007. PMID: 17704234 Free PMC article.
References
-
- Boorse G, Tallman G (1999) Guard cell protoplasts: isolation, culture, and regeneration of plants. In RD Hall, ed, Methods in Molecular Biology: Plant Cell Culture Protocols, Vol. 111. Humana Press, Totawa, NJ, pp 243–257 - PubMed
-
- Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72: 248–254 - PubMed
-
- Burnett EC, Desikan R, Moser RC, Neill SJ (2000) ABA activation of an MBP kinase in Pisum sativum epidermal peels correlates with stomatal responses to ABA. J Exp Bot 51: 197–205 - PubMed
-
- Calderini O, Bögre L, Vicente O, Binarova P, Heberle-Bors E, Wilson C (1998) A cell cycle regulated MAP kinase with a possible role in cytokinesis. J Cell Sci 111: 3091–3100 - PubMed
-
- Carimi F, Zottini M, Formentin E, Terzi M, Schiavo F (2003) Cytokinins: new apoptotic inducers in plants. Planta 216: 413–421 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

