Inducible expression of catalytically active type 1 serine/threonine protein phosphatase in a human carcinoma cell line
- PMID: 12914669
- PMCID: PMC183861
- DOI: 10.1186/1475-2867-3-12
Inducible expression of catalytically active type 1 serine/threonine protein phosphatase in a human carcinoma cell line
Abstract
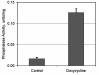
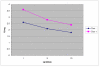
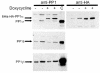
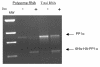
BACKGROUND: One of the major cellular serine/threonine protein phosphatases is protein phosphatase type 1 (PP1). Studies employing many eukaryotic systems all point to a crucial role for PP1 activity in controlling cell cycle progression. One physiological substrate for PP1 appears to be the product of the retinoblastoma susceptibility gene (pRB), a demonstrated tumor suppressor. The growth suppressive activity of pRB is regulated by its phosphorylation state. Of critical importance is the question of the in vivo effect of PP1 activity on pRB and growth regulation. As a first step towards addressing this question, we developed an inducible PP1 expression system to investigate the regulation of PP1 activity. RESULTS: We have established a cell line for inducing protein expression of the type 1, alpha-isotype, serine/threonine protein phosphatase (PP1alpha). A plasmid encoding a fusion protein of the catalytic subunit of PP1alpha with a 6-histidine peptide (6His) and a peptide from hemagluttinin (HA) was transfected into the UMUC3 transitional cell carcinoma cell line, previously transfected with the reverse tetracycline transactivator plasmid pUHD172-1neo. A stable cell line designated LLWO2F was established by selection with hygromycin B. 6His-HA-PP1alpha protein appeared in cell lysates within two hours following addition of doxycycline to the culture medium. This protein localizes to the nucleus as does endogenous PP1alpha, and was shown to associate with PNUTS, a PP1-nuclear targeting subunit. Like endogenous PP1alpha, immunocomplexed 6His-HA-PP1alpha is active toward phosphorylase a and the product of the retinoblastoma susceptibility gene, pRB. When forcibly overexpressing 6His-HA-PP1alpha, there is a concomitant decrease in endogenous PP1alpha levels. CONCLUSIONS: These data suggest the existence of an autoregulatory mechanism by which PP1alpha protein levels and activity remain relatively constant. RT-PCR analyses of isolated polysome fractions support the notion that this putative autoregulatory mechanism is exerted, at least in part, at the translational level. Implications of these findings for the study of PP1alpha function in vivo are discussed.
Figures





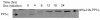

Similar articles
-
The carboxyl-terminal region of the retinoblastoma protein binds non-competitively to protein phosphatase type 1alpha and inhibits catalytic activity.J Biol Chem. 2000 Sep 8;275(36):27784-9. doi: 10.1074/jbc.M004542200. J Biol Chem. 2000. PMID: 10889204
-
Protein phosphatase 1alpha-mediated stimulation of apoptosis is associated with dephosphorylation of the retinoblastoma protein.Oncogene. 2001 Sep 27;20(43):6111-22. doi: 10.1038/sj.onc.1204829. Oncogene. 2001. PMID: 11593419
-
PNUTS (phosphatase nuclear targeting subunit) inhibits retinoblastoma-directed PP1 activity.Biochem Biophys Res Commun. 2002 Sep 27;297(3):463-7. doi: 10.1016/s0006-291x(02)02236-2. Biochem Biophys Res Commun. 2002. PMID: 12270115
-
Control and activity of type-1 serine/threonine protein phosphatase during the cell cycle.Semin Cancer Biol. 1995 Aug;6(4):195-202. doi: 10.1006/scbi.1995.0026. Semin Cancer Biol. 1995. PMID: 8541514 Review.
-
Protein phosphatase 1 is a key player in nuclear events.Cell Signal. 2015 Dec;27(12):2589-98. doi: 10.1016/j.cellsig.2015.08.007. Epub 2015 Aug 11. Cell Signal. 2015. PMID: 26275498 Review.
Cited by
-
A strategy to disentangle direct and indirect effects on (de)phosphorylation by chemical modulators of the phosphatase PP1 in complex cellular contexts.Chem Sci. 2024 Jan 12;15(8):2792-2804. doi: 10.1039/d3sc04746f. eCollection 2024 Feb 22. Chem Sci. 2024. PMID: 38404380 Free PMC article.
References
-
- Ingebritsen TS, Cohen P. Protein phosphatases: properties and role in cellular regulation. Science. 1983;221:331–338. - PubMed
-
- Cyert MS, Thorner J. Putting it on and taking it off: phosphoprotein phosphatase involvement in cell cycle regulation. Cell. 1989;57:891–893. - PubMed
-
- Mumby MC, Walter G. Protein serine/threonine phosphatases: structure regulation, and functions in cell growth. Physiol Rev. 1993;73:673–699. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

