Reovirus receptors and pathogenesis
- PMID: 12915527
- PMCID: PMC187431
- DOI: 10.1128/jvi.77.17.9109-9115.2003
Reovirus receptors and pathogenesis
Figures
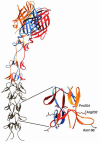
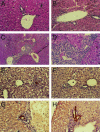
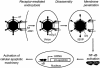
Similar articles
-
Reovirus receptors, cell entry, and proapoptotic signaling.Adv Exp Med Biol. 2013;790:42-71. doi: 10.1007/978-1-4614-7651-1_3. Adv Exp Med Biol. 2013. PMID: 23884585 Free PMC article. Review.
-
The distinct roles of JAM-A in reovirus pathogenesis.Cell Host Microbe. 2009 Jan 22;5(1):3-5. doi: 10.1016/j.chom.2008.12.009. Cell Host Microbe. 2009. PMID: 19154981
-
Structural evidence for common functions and ancestry of the reovirus and adenovirus attachment proteins.Rev Med Virol. 2003 Mar-Apr;13(2):123-32. doi: 10.1002/rmv.379. Rev Med Virol. 2003. PMID: 12627395 Free PMC article. Review.
-
Independent regulation of reovirus membrane penetration and apoptosis by the mu1 phi domain.PLoS Pathog. 2008 Dec;4(12):e1000248. doi: 10.1371/journal.ppat.1000248. Epub 2008 Dec 26. PLoS Pathog. 2008. PMID: 19112493 Free PMC article.
-
Junctional adhesion molecule-A is required for hematogenous dissemination of reovirus.Cell Host Microbe. 2009 Jan 22;5(1):59-71. doi: 10.1016/j.chom.2008.12.001. Cell Host Microbe. 2009. PMID: 19154988 Free PMC article.
Cited by
-
Understanding neurotropic enteric viruses: routes of infection and mechanisms of attenuation.Cell Mol Life Sci. 2024 Oct 4;81(1):413. doi: 10.1007/s00018-024-05450-6. Cell Mol Life Sci. 2024. PMID: 39365457 Free PMC article. Review.
-
Junctional adhesion molecule 1 is a functional receptor for feline calicivirus.J Virol. 2006 May;80(9):4482-90. doi: 10.1128/JVI.80.9.4482-4490.2006. J Virol. 2006. PMID: 16611908 Free PMC article.
-
Manipulation of Host Cell Organelles by Intracellular Pathogens.Int J Mol Sci. 2021 Jun 17;22(12):6484. doi: 10.3390/ijms22126484. Int J Mol Sci. 2021. PMID: 34204285 Free PMC article. Review.
-
Virus-associated disruption of mucosal epithelial tight junctions and its role in viral transmission and spread.Tissue Barriers. 2021 Oct 2;9(4):1943274. doi: 10.1080/21688370.2021.1943274. Epub 2021 Jul 9. Tissue Barriers. 2021. PMID: 34241579 Free PMC article. Review.
-
Virus-Receptor Interactions: The Key to Cellular Invasion.J Mol Biol. 2018 Aug 17;430(17):2590-2611. doi: 10.1016/j.jmb.2018.06.024. Epub 2018 Jun 18. J Mol Biol. 2018. PMID: 29924965 Free PMC article. Review.
References
-
- Azzam-Smoak, K., D. L. Noah, M. J. Stewart, M. A. Blum, and B. Sherry. 2002. Interferon regulatory factor-1, interferon-beta, and reovirus-induced myocarditis. Virology 298:20-29. - PubMed
-
- Banerjea, A. C., K. A. Brechling, C. A. Ray, H. Erikson, D. J. Pickup, and W. K. Joklik. 1988. High-level synthesis of biologically active reovirus protein sigma 1 in a mammalian expression vector system. Virology 167:601-612. - PubMed
-
- Barton, E. S., J. L. Connolly, J. C. Forrest, J. D. Chappell, and T. S. Dermody. 2001. Utilization of sialic acid as a coreceptor enhances reovirus attachment by multistep adhesion strengthening. J. Biol. Chem. 276:2200-2211. - PubMed
-
- Barton, E. S., J. C. Forrest, J. L. Connolly, J. D. Chappell, Y. Liu, F. Schnell, A. Nusrat, C. A. Parkos, and T. S. Dermody. 2001. Junction adhesion molecule is a receptor for reovirus. Cell 104:441-451. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

