A wild-type porcine encephalomyocarditis virus containing a short poly(C) tract is pathogenic to mice, pigs, and cynomolgus macaques
- PMID: 12915530
- PMCID: PMC187386
- DOI: 10.1128/jvi.77.17.9136-9146.2003
A wild-type porcine encephalomyocarditis virus containing a short poly(C) tract is pathogenic to mice, pigs, and cynomolgus macaques
Abstract
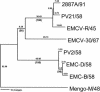
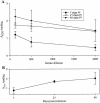
Previous studies using wild-type Encephalomyocarditis virus (EMCV) and Mengo virus, which have long poly(C) tracts (61 to 146 C's) at the 5' nontranslated region of the genome, and variants of these viruses genetically engineered to truncate or substitute the poly(C) tracts have produced conflicting data on the role of the poly(C) tract in the virulence of these viruses. Analysis of the nucleotide sequence of an EMCV strain isolated from an aborted swine fetus (EMCV 30/87) revealed that the virus had a poly(C) tract that was 7- to 10-fold shorter than the poly(C) tracts of other EMCV strains and 4-fold shorter than that of Mengo virus. Subsequently, we investigated the virulence and pathogenesis of this naturally occurring short-poly(C)-tract-containing virus in rodents, pigs, and nonhuman primates. Infection of C57BL/6 mice, pigs, and cynomolgus macaques resulted in similar EMCV 30/87 pathogenesis, with the heart and brain as the primary sites of infections in all three animals, but with different disease phenotypes. Sixteen percent of EMCV 30/87-infected pigs developed acute fatal cardiac failure, whereas the rest of the pigs were overtly asymptomatic for as long as 90 days postinfection (p.i.), despite extensive myocardial and central nervous system (CNS) pathological changes. In contrast, mice infected with >/==" BORDER="0">4 PFU of EMCV 30/87 developed acute encephalitis that resulted in the death of all animals (n = 25) between days 2 and 7 p.i. EMCV 30/87-infected macaques remained overtly asymptomatic for 45 days, despite extensive myocardial and CNS pathological changes and viral persistence in more than 50% of the animals. The short poly(C) tract in EMCV 30/87 (CUC(5)UC(8)) was comparable to that of strain 2887A/91 (C(10)UCUC(3)UC(10)), another recent porcine isolate.
Figures



References
-
- Bae, Y. S., H. M. Eun, and J. W. Yoon. 1989. Genomic differences between diabetogenic and nondiabetogenic variants of encephalomyocarditis virus. Virology 170:282-287. - PubMed
-
- Blanchard, J. L., K. F. Soike, and G. B. Baskin. 1987. Encephalomyocarditis virus infection in African green and squirrel monkeys: comparison of pathological effects. Lab. Anim. Sci. 37:635-639. - PubMed
-
- Brewer, L. A., C. Brown, M. P. Murtaugh, and M. K. Njenga. Transmission of porcine encephalomyocarditis virus (EMCV) to mice by transplanting EMCV-infected pig tissues. Xenotransplantation, in press. - PubMed
-
- Cerutis, D. R., R. H. Bruner, D. C. Thomas, and D. J. Giro. 1989. Tropism and histopathology of the D, B, K and MM variants of encephalomyocarditis virus. J. Med. Virol. 29:63-69. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

